883
The key to understanding insulin resistance
The topic of insulin resistance is very sharp. Most of the diseases of civilization, ranging from depression to cardiovascular disease to Alzheimer's disease is associated with metabolic disorders. However, it is very interesting the links between lifestyle and disease, how nutrients cause specific metabolic disorders.
So today I will tell about FFA – free fatty acids (in the illustrations designated as FFA – free fatty acids). On the one hand, SHC is the primary source of energy, but on the other hand, it is an important signaling molecule.
I want to emphasize that when it comes to chronic increase in FLC as the cause of insulin resistance (IR), remember that the problem is not in FLC, but the fact that they cannot use, so the blood appears at a surplus. The article turned out great, but it is interesting to understand the logic of health and disease – you are welcome.
Free fatty acids: a key to understanding insulin resistance
Free fatty acids.
The availability (or not esterified) fatty acids (FFA) result from hydrolysis of the triglycerides contained in adipose tissue. Plasma fatty acids or esterified in this case, mostly bound to albumin or esterified and are in a free state. Free fatty acids (FFA) are present in lipoproteins of the 5th class.
The latter consist of long-chain fatty acids, is strongly associated with two specific areas of the molecule to albumin; when the level of FFA in plasma, they take the additional parts of the molecule, but the relationship is less strong.
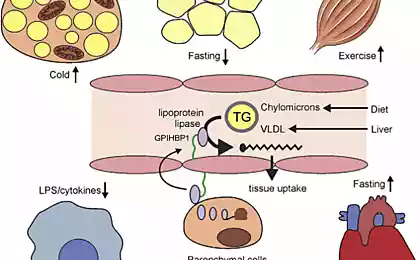
SJK represent the main energy substrate of the body. They are formed during lipolysis of triglycerides accumulated in the fat cells. Tissue lipase in these cells is under neuroendocrine control, and its activation is via the adenylyl cyclase system.
A second source of FFA plasma is the hydrolysis of triglyceride contained in lipoproteins under the influence of lipoprotein lipase. The ability of skeletal muscle (and other tissues) to adjust its metabolism to dominant at the moment, the substrate is called "metabolic good health" or "metabolic flexibility." It is clear that a good "metabolic health" is associated with normal insulin sensitivity.
Exchange of free fatty acids.
The half-life of FLC is quite short — 4 to 8 minutes, and they are easily absorbed from plasma to muscle cells. The second way to exchange them is absorbed by the liver and resynthesis into triglycerides, which can then be transported from the liver in VLDL or oxidized to acetyl COA. Under physiological conditions, the FFA level in the blood can rise and fall very quickly, satisfying the body's need for this form of energy.
Their content is usually lower after the intake of carbohydrates and resulting insulin spike, but the lower the level of blood glucose after eating their level increases. Fasting blood contains typically 400 — 600 mEq/l SJK; with more prolonged fasting (up to 24 — 72 hours) the FFA level can reach 1000 — 1500 µeq/l. Glucagon, epinephrine, growth hormone and ACTH also increase the FFA level. The main physiological regulators of the content of FFA in plasma are insulin and adrenaline.
Every minute is utilized 20-40% FFA into the plasma, they are oxidized, neeterificirovannah or converted into other fatty acids. During rest the oxidation occurs mainly in the liver and heart, and at loads in skeletal muscle, and in the latter case the proportion of oxidized FFA, increases approximately from 20 to 60%.
Most FFA captured by the liver cells, neeterificirovannah with the formation of mainly triglycerides, and phospholipids, synthesis of which is used, typically linoleic acid. Plasma FFA found in the concentration range from 100 µmol/l to 1 mmol/l and their level depends strongly on the time of day.
After each meal the FFA level in the plasma falls because insulin suppresses fat cell lipolysis, which can be formed in FLC. At night, the concentration of FFA in the plasma increases. To this normal daily fluctuations of the levels of FLC "adapt" almost all other tissues, particularly skeletal muscle, which are diverted from disposal of glucose (day) FFA consumption (at night).
Some causes of metabolic FLC.
Key the metabolism of free fatty acids is their chronic promotion. Chronic hanging of FFA level can be caused by many reasons food and stress type. For example, excess of carbohydrates, which starts the synthesis of new fatty acids from excess carbohydrates.
Because in a healthy person 75-80% of glucose is utilized by skeletal muscle, physical activity working heavy labor can prevent the development of functional resistance to insulin. Lack of physical activity also causes an increase of FLC.
Chronic stress of any type is another cause of chronic high FFA. During times of stress in the blood increases the FFA level to ensure the functioning of the heart and muscles. But people are usually not moving and all these fatty acids circulate in the blood for a long time.
In large-scale studies found an inverse relationship between position on the socio-economic ladder and the chance of developing metabolic syndrome. It is concluded that the development of metabolic syndrome – a biological mechanism leading to "social inequality in coronary risk among men."
High coronary risk (elevated triglycerides and low cholesterol-high density lipoproteins) are associated with low socio-economic status ("poor child"), which in later years leads to overweight and obesity.
In addition, psychological stress also contribute to the development of T1DM and T2DM, as a high level of FLC is associated with increased formation of mitochondrial reactive oxygen species (oxidative stress), which ultimately leads to a reduced ability of cells to respond adequately to the action of insulin.
The FLC and the metabolite and signaling molecule.
Numerous studies in recent years have shown that, first, the FLC is not only a high-energy fuel, but also important signal molecules. Their concentration is an important regulatory factor affecting the rate of glucose utilization in the muscles.
And, second, adipose tissue is an important endocrine organ that secretes a large number of factors, called adipocytokines that have or sensitizing effect on insulin (it's adiponectin and leptin), in particular, tumor necrosis factor – alpha (TNF - alpha), resistin, etc.
When excess amounts of adipose tissues, there is excessive lipolysis. Normally, the release of FFA from adipose tissue is strictly regulated, which ensures that other tissues are clearly balanced amount of FFA needed to adequately meet their energy needs.
But with obesity in the bloodstream, thus, pathologically increased amounts of signaling molecules (especially TNF-alpha), leading to disruption of metabolic homeostasis. Thus, the early events leading to disruption of the mechanism of action of insulin and the occurrence of IR, occur in adipose cells and long before the emergence of disturbed glucose tolerance.
The profile of free fatty acids in the serum varies with age and gender, diet, changes in hormonal status; for example, adrenalectomy or castration lead to changes in the profile of the LCD.
The change in the concentration of free LC in turn leads to significant changes in two stages transfer of hormonal information, the binding of glucocorticoids with their specific carrier proteins in the plasma and tissue receptors.
With modern positions free fatty acids are viewed not as passive substrates involved in metabolic processes. Free fatty acids are important metabolic signals and participants lipid disorders. In some situations, they behave like hormone-like molecules influencing the transcription of genes by binding with several receptors.
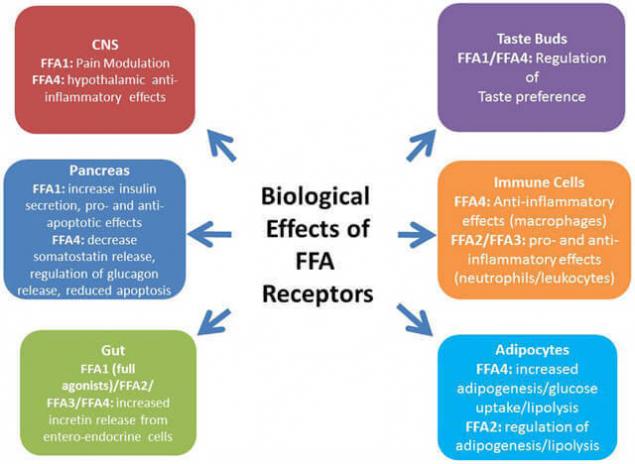
Among the receptors, which are associated with FFA/FFA or their derivatives, occupy a special place with peroksisom the chervyachey-activated receptors – PPARs. Also coupled with G-protein receptor GPR120, which in large quantities is expressed in intestine, functions as a receptor for unsaturated long-chain free fatty acids FFA. Stimulation of GPR120 by free fatty acids promotes the secretion of GLP-1 and increases the number of circulating insulin.
Free fatty acids and health.
In foreign medical literature free fatty acids (FFA) or nonesterified fatty acids, which are formed by hydrolysis of tri-glycerides contained in the adipose tissue, referred to as "red light on the dashboard of a myocardium".
Increase their plasma levels of signals of increasing danger: the beginning of metabolic syndrome, then insulin resistance, diabetic cardiomyopathy, and later coronary heart disease. Further, this light bulb can burn out and break out the "light" markers of myocardial necrosis, indicating that "point of no return" is passed.
Thus, elevated levels of FFA-directly related to obesity, i.e., "these bulbs start to glow long before the development of endpoint", which makes possible the effective implementation of corrective actions.
Numerous recent studies clearly indicate elevated levels of FLC caused by excessive amount of fatty tissue – if not the first, then at least one of the main causes of IR. Repeatedly and reliably shown that most patients suffering from obesity, metabolic syndrome and diabetes of the second type (type 2), have elevated levels of FLC, which leads to IL-many tissues – adipose, muscle, liver, and endothelial cells.
FFA is an independent predictor of violations glucose tolerance and type 2 diabetes. So, the increased flow of FFA from the great mass of the adipose cells and also irregularities in the mechanisms of storage of triglycerides in the mechanisms of lipolysis in the tissue, the source is normally sensitive to insulin, it would appear that the earliest manifestations of anomalies, leading to IR.
It is essential that these violations are detected before the development of postprandial hyperglycemia, or before the development of hyperglycemia on an empty stomach. Indeed, the increase in fasting plasma levels of FFA, perhaps, the earliest indication of any future breach of tolerance to glucose.
The ladder of insulinorezistentnost.
Step one: violation latinoboy regulation (leptinresistance).
In the distribution of fatty acids in the human body involved mainly two hormones: growth hormone controls the mobilization of fatty acids from adipose tissue, and leptin, which controls β-oxidation of fatty acids in mitochondria.
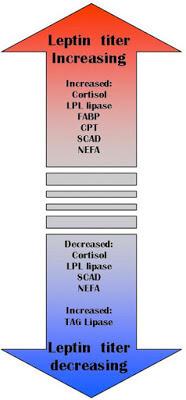
An important function of leptin is the retention of triglycerides in adipocytes. Normal levels of leptin protects other organs from fat accumulation (blood vessels, liver, muscle, etc.). Leptin activates carnitine Palmitoyl-Shuttle zu-1, which binds the fatty acid from carnitine, while the latter carries it through the membrane of mitochondria, and this process is strictly regulated. Leptin also stimulates fatty acid oxidation and reduces the amount of triacylglycerols in muscle tissue. It is also established that leptin inhibits the activity of acetyl-COA-carboxylase!
Chronic stress, overeating, malnutrition, excess sugar, lack of exercise lead to the disruption of latinoboy system. If there is a resistance to leptin, it leads to an increase in the number of free fatty acids. Comes the second step – a chronic increase in FLC.
Step two: increase in visceral adipose tissue.
It visceral fat tissue is a powerful source of FLC. Visceral fat increases in proportion to the body mass index is an independent predictor of developing diabetes type 2 diabetes. Visceral adipose tissue is a major source of free fatty acids (FFA).
In visceral obesity the liver via the portal vein receives excessive (20 to 30 times greater than normal) amount of free fatty acids, exposing the liver to severe strains and eventually leads to the development of the above metabolic disorders.
Visceral fat that is present around internal organs, the mesentery and the omentum, differs from the type of subcutaneous adipocytes, their endocrine function, lipolytic activity, insulin sensitivity and other hormones.
Unlike subcutaneous adipose tissue, venous blood, flowing from visceral fat through the portal system directly enters the liver. This makes direct delivery to the liver of great amount of free fatty acids (FFA) and adipokines, can synthesize - ing in visceral adipose tissue.
Adipokines in turn activate hepatic immune mechanisms leading to the formation of proinflammatory mediators, such as C-reactive protein (CRP) and others. Free fatty acids in a large number of coming into the liver from visceral adipose tissue, contributes to the development of hepatic insulin resistance.
Chemoattractant secreting monocyte protein-1 (MCP-1), contributing to macrophage infiltration of adipose tissue, adipocytes contribute to the proinflammatory state.
Macrophages, in turn, represent an important source of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Visceral adipose tissue is characterized by greater infiltration vespoli - tive cells, and therefore secretes large quantities of inflammatory cytokines than subcutaneous fat
Ectopic fat, i.e. fat that is not subcutaneous adipose tissue. This fat is often visceral, or hepatic, or intramuscular. But it shares something that is not subcutaneous fat, and those with the "wrong" place. Further disease development will depend on what tissues not designed for storage, will accumulate FLC. If they accumulate in skeletal muscle – this will result IR, if the liver to dyslipidaemia. First, as a rule, develop IR, then, with its weighting – ischemic heart disease – coronary heart disease.
Step three: chronic increase of FLC.
As shown above, in visceral obesity the liver via the portal vein receives excessive (20 to 30 times greater than normal) amount of free fatty acids, exposing the liver to severe strains and eventually leads to the development of the above metabolic disorders. Blood appears chronic elevated levels of FLC. Together with leptinresistance this gradually leads to an increase in the amount of fat in non-fat organs.
Under the action of FFA in the adipose tissue formed of larger adipocytes that are resistant to the action of insulin, it triggers a process of local inflammation, increased secretion of proinflammatory cytokines.
Chronic increase in the level of fatty acids in the blood is a consequence of disturbances in the body systems regulation of their homeostasis. Resistance to leptin, apparently, does not allow exceeding certain its stationary limit oxidation, and therefore, the disposal of excess amounts of fatty acids in mitochondria.
So, as you can guess, the situation arises when, due to the high content of fatty acids in the extracellular space, their flux into the cell increases (due to increasing the content of fatty acids in the body because of their nedorazumeniya), and while resistance to the leptin oxidation of fatty acids remains unchanged.
Perhaps an inverse relationship between intake of fatty acids in the cell nairboi tissue and their secretion into the blood or is disturbed, or it does not exist, i.e. there is no mechanism for maintaining stationary levels of fatty acids in the blood. If this assumption is true, then in this respect the regulation of the metabolism of fatty acids is fundamentally different from the regulation of glucose metabolism, the stationary level of which is maintained by hormones.
Under control is probably the only fatty acid oxidation in the mitochondria, i.e. the intracellular utilization of this energy substrate. In recent years we have studied the mechanisms of regulation of the flow of fatty acids in the body.
Was revealed to the family of nuclear receptors PPAR, they became known in connection with the ability to induce proliferation by peroxisome and carcinogenesis in the liver in response to exposure to xenobiotics. There are three isoforms, PPAR— α, γ and δ, and the most studied properties. tori PPARα and Ppary. Ligands for receptors serve saturated, unsaturated and monounsaturated fatty acids.
Ppary is expressed in adipocytes and reduces the secretion of fatty acids into blood from adipose tissue. PPARα is expressed in liver cells, skeletal and cardiac muscle and acts as a "lipostat", regulating the processes of intracellular synthesis and β-oxidation of fatty acids in mitochondria and peroxisome.
PPAR is stimulated by leptin, growth hormone and insulin, their expression is subject to a circadian rhythm that they expressed in response to the meal. These receptors carry out intracellular regulation of fatty acids, maintaining a fixed level of consumption of energy by the cell, but they apparently still do not participate in maintaining the homeostasis of fatty acids at the level of the body.
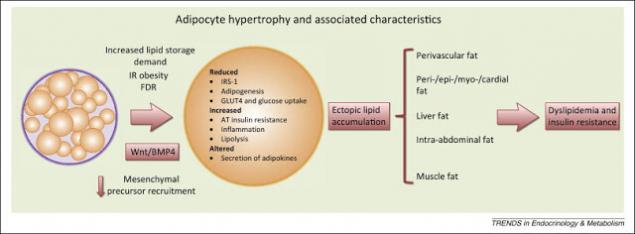
Step four: the emergence of insulinrezistentnost.
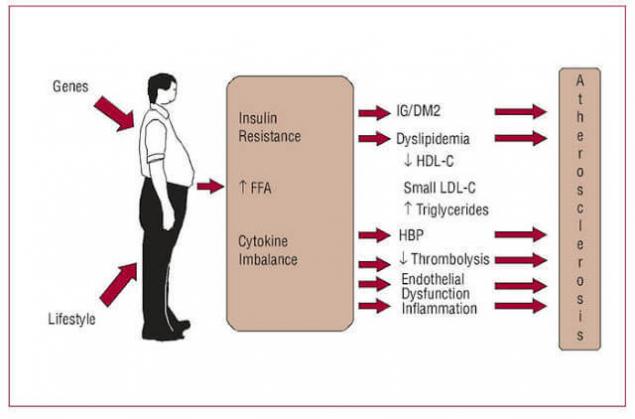
Elevated levels of FFA leads to their accumulation in the cells, rebuilding cell membranes and reduce insulin resistance. An excess of triglycerides in the cells causes increased synthesis of inflammatory cytokines. That is fat tissue is currently considered as the place of the original occurrence and development of IR. This is due to: a) arrivals into the bloodstream elevated levels of FFA, and, 2) increased secretion adipocytokine.
A large mass of adipocytes synthesize increased amounts of proinflammatory cytokines, which leads to a chronic inflammatory process which: a) violates the transmission of the insulin signal and, b) impairs mitochondrial function, which violates the homeostasis of glucose. In particular, secreted by fat cells, IL-6 and TNF-alpha weight of IR, and secreted angiotensin II, increases blood pressure and promotes atherosclerosis.
Violation of adaptive mechanisms.
In the cell from fatty acids, not used for β-oxidation, the phospholipids are synthesized first, and then triglycerides, which accumulate in the cytoplasm. Intracellular triglycerides in non-fatty tissues contain mainly palmitic acid. Of palmitic acid is synthesized sphingomyelin, which is a major component of membrane rafts, involved in regulating the activity of membrane receptors.
The synthesis of sphingomyelin, depending on the content in the cell palmitin howling acid, by way of "palmitic acid → ceramide → sphingomyelin". It is the way of synthesis of ceramide from palmitic acid leads to oxidative apoptosis. Ceramide is an inducer of apoptosis as in the oxidative pathway (ceramide inhibits complex III of the etc, causing an enhanced generation of oxidants), and without the involvement of mitochondria.
The accumulation of triglycerides in cardiomyocytes is associated with a reduction of cardiolipin synthesis and the change in the respiratory function of mitochondria, since cytochrome C oxidase of the complex IV of ETS is associated with cardiolipin. Changing the structure of the mitochondrial membrane leads to the release of cytochrome C and apoptosis without the involvement of oxidants. Thus, the accumulation of palmitic acid in the cells of non-fat tissue leads to increased synthesis of ceramide and the decreased synthesis of cardiolipin that induces apoptosis, and altering the activity of receptors.
In this regard, the accumulation in the cells of triglycerides (triglycerides not induce apoptosis) is considered as the body's attempt to avoid the effect of lipotoxicity.
Sphingomyelin, and palmitic acid exhibit a high affinity for cholesterol. Increase in content of sphingomyelin, and palmitic acid in the membrane may explain associated with age accumulation in membranes of cholesterol and sensitivity of insulin receptor.
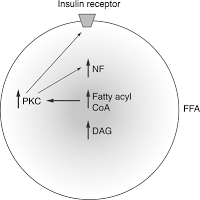
The insulin receptor is associated with membrane rafts, and changes in the composition of rafts affects its sensitivity. Accumulation of triglycerides in non-fat tissues and associated reduced sensitivity of the insulin receptor leads to insulin resistance and hyperglycemia, i.e. increased content of glucose in the blood. Receptor to insulin is tirozinkinazy.
Autophosphorylation are activated through various ways, in particular, the path PI-3-K (phosphoinositol-3-Kenaz), which is the transport of glucose inside the cell, as it comes in its active operating state of the glucose Transporter GLUT4.
Due to active lipolysis of free fatty acids (FFA) and proinflammatory cytokines, they affect substrates of the insulin receptor and thereby blocks the path PI-3-K, resulting in blocked effects this way on the glucose metabolism, and glucose cannot enter the cell. In this way develops insulin resistance, i.e. an excessive amount of visceral adipose tissue inhibits insulin signal and causes insulin receptors become insensitive to insulin and its biological role is perverted.
Depending on individual sensitivity and genetics, insulin resistance can develop in different tissues. The abundance of FLC mediates the progression of insulin resistance in many tissues — muscle, including myocardial, hepatic, adipose and endothelial cells, promotes the progression of ischemic changes in the myocardium, including changes related to violation of the beta-oxidation of FFA to the myocardium.
Step five: hepatic and muscle insulin resistance.
In conditions of high intake of food rich in fats and carbohydrates, stimulates insulin secretion, which in turn activates lipogenesis and deposition of SFA in the adipose tissue. However, there are genetically-determined limit the ability to accumulate lipids, so when the amount of adipose tissue reaches its maximum, the excess FLC begins to flow into the liver and muscles.
The abundance of FLC is accompanied by accumulation of triglycerides in parenchymal cells of many tissues, namely skeletal and cardiac myocytes and hepatocytes, which leads to their damage and chronic dysfunction.
As a result, the liver under conditions of insulin resistance begins to actively synthesize fatty acids, triglycerides, accelerated lipolysis, but in adipose tissue. In addition, in the liver occur all those processes that lead the patient with visceral obesity to diabetes: stimulates gluconeogenesis and inhibiting glycolysis and glycogen synthesis.
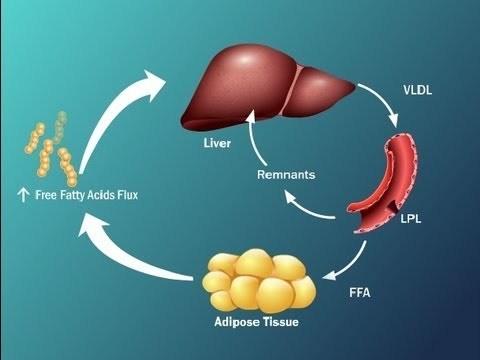
Clarified a rather complex plan, which reflects that insulin resistance, or insulin resistance, leads to the fact that the liver is becoming overwhelmed fatty acids.
This is due to the fact that the synthesized active fatty acid in the liver, decreased oxidation of fatty acids, are actively fatty acids to the liver from visceral adipose tissue and, in addition, the fat in the composition of chylomicrons is supplied to us in the liver, also overloads the liver the free fatty acids.
These processes lead to the fact that the liver is unable to metabolize by β-oxidation of FFA-activated lipid peroxidation, resulting in large quantities are produced by reactive oxygen species, oxidative stress occurs and that these factors lead to the phosphorylation of a substrate of the insulin receptor, what we talked about on the previous slide, thereby again starts the insulin resistance, i.e. a kind of vicious circle, and to determine the patient that is primary is difficult.
It has been shown that macrophages of visceral adipose tissue have anti-inflammatory activity. Also in adipose tissue was found and CD 8 + T-lymphocytes that actively secrete cytokines provospalitelna and, thus, liver steatosis can already move to the next stage with the development in patients of Nash and other consequences, which leads to NAFLD.
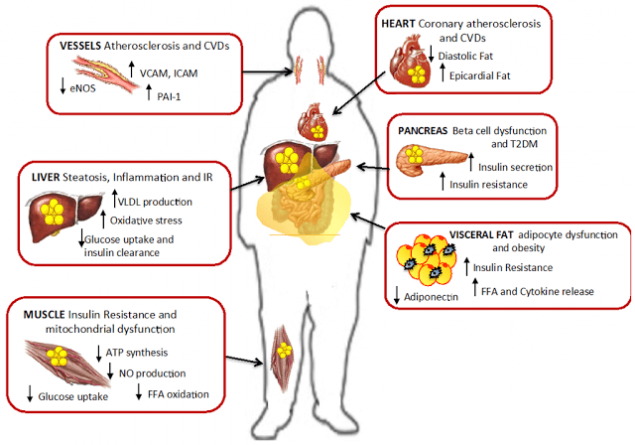
Muscle insulin resistance.
Soon after the liver, fat begins to accumulate in the muscles. Characteristic IR metabolic pathology is accumulation around muscle fibrils triglycerides. However, the accumulation of triglycerides within skeletal muscle, as expected, is not the direct cause of the development of type 2 diabetes, but similar. may be marker of lipid intermediates. such as acetyl COA, ceramides and diacylglycerol
According to a recent study, impaired transmission of the insulin signal is associated mostly with abnormal FFA metabolism in cells of skeletal muscle, "not coping" with their recycling, when FLC in excess. Indeed, local accumulation within skeletal muscle FFA metabolites such as ceramides, diglycerol or acyl-COA leads to impaired transmission of the insulin signal and, thus, to disruption of glucose transport.
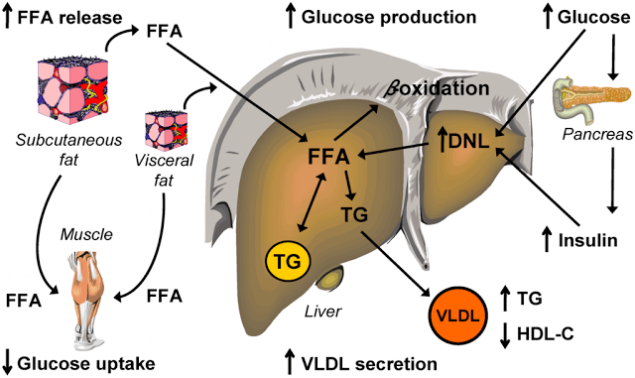
Step six. The vicious cycle of insulin resistance.
Insulin resistance caused by high FFA level, further increases the concentration of FLC in plasma. It had been revealed that insulin-resistant fat cells secrete elevated levels of FLC. It is, in fact, allows to consider elevated levels of FLC marker of IR.
Indeed, when IRA level of FFA in hepatocytes is increased, because, they:
1) increased de novo lipogenesis,
2) eterificare FLC exceeds their oxidation,
3) esterified LCD stored as triglycerides or are directed to the synthesis of X-VLDL (rich in triglycerides),
4) adjustable insulin decreases mobilization of triglycerides.
Insulin-resistant adipocytes is intensively contained in them break down triglycerides and release of FFA formed them into the bloodstream (as in obesity, and without it). The flow of FFA from fat cells is increased and, moreover, FFA also come from X-VLDL and chylomicrons from plasma and blood were being used in other bodies, and partly back to the liver where it is again converted to triglycerides. There is a "pumping" of the liver, FFA, and triglycerides. It has the most serious consequences.
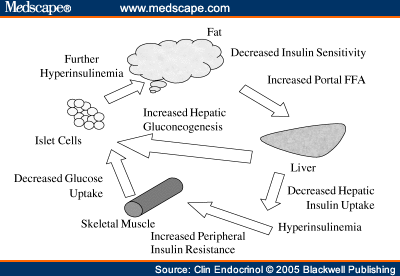
Step seven. Acceleration of atherosclerosis.
Elevated levels of FFA leads to dyslipidemia and atherogenesis
How insulin resistance leads to dyslipidemia Increase in liver triglycerides stimulates the formation of APO and X-VLDL,
This is how it happens.
1) From the liver to high levels of X-VLDL secreted into the plasma, where due to lipolysis from X-VLDL are formed of FLC and vysokoyarusnye remnant (residual) particles of lipoproteins rich in triglycerides.
2) From plasma FFA and remnant particles are again absorbed by the liver, which further increases the level of FFA in the hepatocytes and further stimulates the synthesis of X-VLDL.
3) In the liver, with a high level X-VLDL normal level of protein CETP (cholesteryl ester transfer protein) is a Transporter of cholesterol ester, triglycerides of X-VLDL are transferred in the X-HDL, and cholesterol from X-HDL is transferred in the X-VLDL. In the end, consist of: a) very cholesterol-rich atherogenic particles remnant X-VLDL, and b) X-HDL, containing a lot of triglycerides and a little cholesterol.
4) These particles X-HDL lose triglycerides (hepatic lipase) and their main apolipoprotein, APO A1. In the end, the level antiaterogennoe X-HDL is lowered.
5) At high levels of X-VLDL (rich in triglycerides), CETP transfers triglycerides from X-VLDL, S-LDL, and cholesterol from X-X in LDL-VLDL.
6) Rich in triglycerides, S-LDL because of the activity of hepatic or lipoprotein lipase, lose triglycerides, decrease in size and become highly atherogenic small dense particles X-LDL.
Thus, elevated levels of FFA reduce the level of "antiatherogenic" X-HDL, the formation of highly atherogenic small dense particles X -, LDL-cholesterol and increased plasma triglyceride levels.
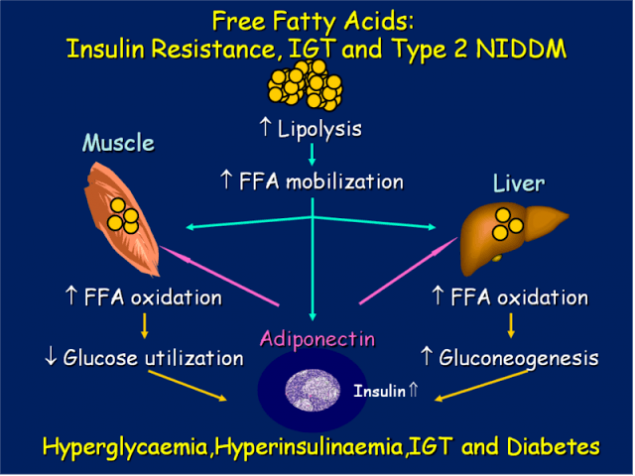
But there is another way in which high levels of FFA induce atherogenesis. This path is more direct and short. When IL increased the level of FFA in the mitochondria causes macrovascular endothelial cells surgentes of reactive oxygen species, leading to oxidation of h-LDL and modification of X-HDL. It induces an inflammatory process in the vessel walls, the formation and accumulation of plaque and results in ischemia.published
Sources.
1. The Publication Of V. V. Velkova
2. Vladimir Velkov. Laboratory diagnosis. – 2009. Vol. 3, No. 49. – P 55 to 71.
3. Velkov V. V. the Free fatty acids as a risk factor for insulin resistance and ischemia: prospects for risk assessment and diagnosis
4. L. I. Danilova Phenomenon of insulin resistance in clinical practice: mechanisms of formation and possibilities of correction
5. Tereshina E. V. the Role of fatty acids in the development of age-related oxidative stress. Advances in gerontology, 2007. Vol. 20, No. 1
6. "Endocrinology and metabolism", F. Felig, D. Baxter
Author: Andrey Blueskin
Psychosomatics diseases of the neck, Through the throat we "swallow" the reality
We shouldn'T treat
Source: www.beloveshkin.com/2016/01/svobodnye-zhirnye-kisloty-klyuch-k-ponimaniyu-insulinorezistentnosti.html
So today I will tell about FFA – free fatty acids (in the illustrations designated as FFA – free fatty acids). On the one hand, SHC is the primary source of energy, but on the other hand, it is an important signaling molecule.
I want to emphasize that when it comes to chronic increase in FLC as the cause of insulin resistance (IR), remember that the problem is not in FLC, but the fact that they cannot use, so the blood appears at a surplus. The article turned out great, but it is interesting to understand the logic of health and disease – you are welcome.

Free fatty acids: a key to understanding insulin resistance
Free fatty acids.
The availability (or not esterified) fatty acids (FFA) result from hydrolysis of the triglycerides contained in adipose tissue. Plasma fatty acids or esterified in this case, mostly bound to albumin or esterified and are in a free state. Free fatty acids (FFA) are present in lipoproteins of the 5th class.
The latter consist of long-chain fatty acids, is strongly associated with two specific areas of the molecule to albumin; when the level of FFA in plasma, they take the additional parts of the molecule, but the relationship is less strong.
SJK represent the main energy substrate of the body. They are formed during lipolysis of triglycerides accumulated in the fat cells. Tissue lipase in these cells is under neuroendocrine control, and its activation is via the adenylyl cyclase system.
A second source of FFA plasma is the hydrolysis of triglyceride contained in lipoproteins under the influence of lipoprotein lipase. The ability of skeletal muscle (and other tissues) to adjust its metabolism to dominant at the moment, the substrate is called "metabolic good health" or "metabolic flexibility." It is clear that a good "metabolic health" is associated with normal insulin sensitivity.
Exchange of free fatty acids.
The half-life of FLC is quite short — 4 to 8 minutes, and they are easily absorbed from plasma to muscle cells. The second way to exchange them is absorbed by the liver and resynthesis into triglycerides, which can then be transported from the liver in VLDL or oxidized to acetyl COA. Under physiological conditions, the FFA level in the blood can rise and fall very quickly, satisfying the body's need for this form of energy.
Their content is usually lower after the intake of carbohydrates and resulting insulin spike, but the lower the level of blood glucose after eating their level increases. Fasting blood contains typically 400 — 600 mEq/l SJK; with more prolonged fasting (up to 24 — 72 hours) the FFA level can reach 1000 — 1500 µeq/l. Glucagon, epinephrine, growth hormone and ACTH also increase the FFA level. The main physiological regulators of the content of FFA in plasma are insulin and adrenaline.
Every minute is utilized 20-40% FFA into the plasma, they are oxidized, neeterificirovannah or converted into other fatty acids. During rest the oxidation occurs mainly in the liver and heart, and at loads in skeletal muscle, and in the latter case the proportion of oxidized FFA, increases approximately from 20 to 60%.
Most FFA captured by the liver cells, neeterificirovannah with the formation of mainly triglycerides, and phospholipids, synthesis of which is used, typically linoleic acid. Plasma FFA found in the concentration range from 100 µmol/l to 1 mmol/l and their level depends strongly on the time of day.
After each meal the FFA level in the plasma falls because insulin suppresses fat cell lipolysis, which can be formed in FLC. At night, the concentration of FFA in the plasma increases. To this normal daily fluctuations of the levels of FLC "adapt" almost all other tissues, particularly skeletal muscle, which are diverted from disposal of glucose (day) FFA consumption (at night).
Some causes of metabolic FLC.
Key the metabolism of free fatty acids is their chronic promotion. Chronic hanging of FFA level can be caused by many reasons food and stress type. For example, excess of carbohydrates, which starts the synthesis of new fatty acids from excess carbohydrates.
Because in a healthy person 75-80% of glucose is utilized by skeletal muscle, physical activity working heavy labor can prevent the development of functional resistance to insulin. Lack of physical activity also causes an increase of FLC.
Chronic stress of any type is another cause of chronic high FFA. During times of stress in the blood increases the FFA level to ensure the functioning of the heart and muscles. But people are usually not moving and all these fatty acids circulate in the blood for a long time.
In large-scale studies found an inverse relationship between position on the socio-economic ladder and the chance of developing metabolic syndrome. It is concluded that the development of metabolic syndrome – a biological mechanism leading to "social inequality in coronary risk among men."
High coronary risk (elevated triglycerides and low cholesterol-high density lipoproteins) are associated with low socio-economic status ("poor child"), which in later years leads to overweight and obesity.
In addition, psychological stress also contribute to the development of T1DM and T2DM, as a high level of FLC is associated with increased formation of mitochondrial reactive oxygen species (oxidative stress), which ultimately leads to a reduced ability of cells to respond adequately to the action of insulin.
The FLC and the metabolite and signaling molecule.
Numerous studies in recent years have shown that, first, the FLC is not only a high-energy fuel, but also important signal molecules. Their concentration is an important regulatory factor affecting the rate of glucose utilization in the muscles.
And, second, adipose tissue is an important endocrine organ that secretes a large number of factors, called adipocytokines that have or sensitizing effect on insulin (it's adiponectin and leptin), in particular, tumor necrosis factor – alpha (TNF - alpha), resistin, etc.
When excess amounts of adipose tissues, there is excessive lipolysis. Normally, the release of FFA from adipose tissue is strictly regulated, which ensures that other tissues are clearly balanced amount of FFA needed to adequately meet their energy needs.
But with obesity in the bloodstream, thus, pathologically increased amounts of signaling molecules (especially TNF-alpha), leading to disruption of metabolic homeostasis. Thus, the early events leading to disruption of the mechanism of action of insulin and the occurrence of IR, occur in adipose cells and long before the emergence of disturbed glucose tolerance.

The profile of free fatty acids in the serum varies with age and gender, diet, changes in hormonal status; for example, adrenalectomy or castration lead to changes in the profile of the LCD.
The change in the concentration of free LC in turn leads to significant changes in two stages transfer of hormonal information, the binding of glucocorticoids with their specific carrier proteins in the plasma and tissue receptors.
With modern positions free fatty acids are viewed not as passive substrates involved in metabolic processes. Free fatty acids are important metabolic signals and participants lipid disorders. In some situations, they behave like hormone-like molecules influencing the transcription of genes by binding with several receptors.
Among the receptors, which are associated with FFA/FFA or their derivatives, occupy a special place with peroksisom the chervyachey-activated receptors – PPARs. Also coupled with G-protein receptor GPR120, which in large quantities is expressed in intestine, functions as a receptor for unsaturated long-chain free fatty acids FFA. Stimulation of GPR120 by free fatty acids promotes the secretion of GLP-1 and increases the number of circulating insulin.
Free fatty acids and health.
In foreign medical literature free fatty acids (FFA) or nonesterified fatty acids, which are formed by hydrolysis of tri-glycerides contained in the adipose tissue, referred to as "red light on the dashboard of a myocardium".
Increase their plasma levels of signals of increasing danger: the beginning of metabolic syndrome, then insulin resistance, diabetic cardiomyopathy, and later coronary heart disease. Further, this light bulb can burn out and break out the "light" markers of myocardial necrosis, indicating that "point of no return" is passed.
Thus, elevated levels of FFA-directly related to obesity, i.e., "these bulbs start to glow long before the development of endpoint", which makes possible the effective implementation of corrective actions.
Numerous recent studies clearly indicate elevated levels of FLC caused by excessive amount of fatty tissue – if not the first, then at least one of the main causes of IR. Repeatedly and reliably shown that most patients suffering from obesity, metabolic syndrome and diabetes of the second type (type 2), have elevated levels of FLC, which leads to IL-many tissues – adipose, muscle, liver, and endothelial cells.
FFA is an independent predictor of violations glucose tolerance and type 2 diabetes. So, the increased flow of FFA from the great mass of the adipose cells and also irregularities in the mechanisms of storage of triglycerides in the mechanisms of lipolysis in the tissue, the source is normally sensitive to insulin, it would appear that the earliest manifestations of anomalies, leading to IR.
It is essential that these violations are detected before the development of postprandial hyperglycemia, or before the development of hyperglycemia on an empty stomach. Indeed, the increase in fasting plasma levels of FFA, perhaps, the earliest indication of any future breach of tolerance to glucose.

The ladder of insulinorezistentnost.
Step one: violation latinoboy regulation (leptinresistance).
In the distribution of fatty acids in the human body involved mainly two hormones: growth hormone controls the mobilization of fatty acids from adipose tissue, and leptin, which controls β-oxidation of fatty acids in mitochondria.

An important function of leptin is the retention of triglycerides in adipocytes. Normal levels of leptin protects other organs from fat accumulation (blood vessels, liver, muscle, etc.). Leptin activates carnitine Palmitoyl-Shuttle zu-1, which binds the fatty acid from carnitine, while the latter carries it through the membrane of mitochondria, and this process is strictly regulated. Leptin also stimulates fatty acid oxidation and reduces the amount of triacylglycerols in muscle tissue. It is also established that leptin inhibits the activity of acetyl-COA-carboxylase!
Chronic stress, overeating, malnutrition, excess sugar, lack of exercise lead to the disruption of latinoboy system. If there is a resistance to leptin, it leads to an increase in the number of free fatty acids. Comes the second step – a chronic increase in FLC.
Step two: increase in visceral adipose tissue.
It visceral fat tissue is a powerful source of FLC. Visceral fat increases in proportion to the body mass index is an independent predictor of developing diabetes type 2 diabetes. Visceral adipose tissue is a major source of free fatty acids (FFA).
In visceral obesity the liver via the portal vein receives excessive (20 to 30 times greater than normal) amount of free fatty acids, exposing the liver to severe strains and eventually leads to the development of the above metabolic disorders.
Visceral fat that is present around internal organs, the mesentery and the omentum, differs from the type of subcutaneous adipocytes, their endocrine function, lipolytic activity, insulin sensitivity and other hormones.
Unlike subcutaneous adipose tissue, venous blood, flowing from visceral fat through the portal system directly enters the liver. This makes direct delivery to the liver of great amount of free fatty acids (FFA) and adipokines, can synthesize - ing in visceral adipose tissue.
Adipokines in turn activate hepatic immune mechanisms leading to the formation of proinflammatory mediators, such as C-reactive protein (CRP) and others. Free fatty acids in a large number of coming into the liver from visceral adipose tissue, contributes to the development of hepatic insulin resistance.
Chemoattractant secreting monocyte protein-1 (MCP-1), contributing to macrophage infiltration of adipose tissue, adipocytes contribute to the proinflammatory state.
Macrophages, in turn, represent an important source of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Visceral adipose tissue is characterized by greater infiltration vespoli - tive cells, and therefore secretes large quantities of inflammatory cytokines than subcutaneous fat
Ectopic fat, i.e. fat that is not subcutaneous adipose tissue. This fat is often visceral, or hepatic, or intramuscular. But it shares something that is not subcutaneous fat, and those with the "wrong" place. Further disease development will depend on what tissues not designed for storage, will accumulate FLC. If they accumulate in skeletal muscle – this will result IR, if the liver to dyslipidaemia. First, as a rule, develop IR, then, with its weighting – ischemic heart disease – coronary heart disease.
Step three: chronic increase of FLC.
As shown above, in visceral obesity the liver via the portal vein receives excessive (20 to 30 times greater than normal) amount of free fatty acids, exposing the liver to severe strains and eventually leads to the development of the above metabolic disorders. Blood appears chronic elevated levels of FLC. Together with leptinresistance this gradually leads to an increase in the amount of fat in non-fat organs.
Under the action of FFA in the adipose tissue formed of larger adipocytes that are resistant to the action of insulin, it triggers a process of local inflammation, increased secretion of proinflammatory cytokines.
Chronic increase in the level of fatty acids in the blood is a consequence of disturbances in the body systems regulation of their homeostasis. Resistance to leptin, apparently, does not allow exceeding certain its stationary limit oxidation, and therefore, the disposal of excess amounts of fatty acids in mitochondria.
So, as you can guess, the situation arises when, due to the high content of fatty acids in the extracellular space, their flux into the cell increases (due to increasing the content of fatty acids in the body because of their nedorazumeniya), and while resistance to the leptin oxidation of fatty acids remains unchanged.
Perhaps an inverse relationship between intake of fatty acids in the cell nairboi tissue and their secretion into the blood or is disturbed, or it does not exist, i.e. there is no mechanism for maintaining stationary levels of fatty acids in the blood. If this assumption is true, then in this respect the regulation of the metabolism of fatty acids is fundamentally different from the regulation of glucose metabolism, the stationary level of which is maintained by hormones.
Under control is probably the only fatty acid oxidation in the mitochondria, i.e. the intracellular utilization of this energy substrate. In recent years we have studied the mechanisms of regulation of the flow of fatty acids in the body.
Was revealed to the family of nuclear receptors PPAR, they became known in connection with the ability to induce proliferation by peroxisome and carcinogenesis in the liver in response to exposure to xenobiotics. There are three isoforms, PPAR— α, γ and δ, and the most studied properties. tori PPARα and Ppary. Ligands for receptors serve saturated, unsaturated and monounsaturated fatty acids.
Ppary is expressed in adipocytes and reduces the secretion of fatty acids into blood from adipose tissue. PPARα is expressed in liver cells, skeletal and cardiac muscle and acts as a "lipostat", regulating the processes of intracellular synthesis and β-oxidation of fatty acids in mitochondria and peroxisome.
PPAR is stimulated by leptin, growth hormone and insulin, their expression is subject to a circadian rhythm that they expressed in response to the meal. These receptors carry out intracellular regulation of fatty acids, maintaining a fixed level of consumption of energy by the cell, but they apparently still do not participate in maintaining the homeostasis of fatty acids at the level of the body.

Step four: the emergence of insulinrezistentnost.
Elevated levels of FFA leads to their accumulation in the cells, rebuilding cell membranes and reduce insulin resistance. An excess of triglycerides in the cells causes increased synthesis of inflammatory cytokines. That is fat tissue is currently considered as the place of the original occurrence and development of IR. This is due to: a) arrivals into the bloodstream elevated levels of FFA, and, 2) increased secretion adipocytokine.
A large mass of adipocytes synthesize increased amounts of proinflammatory cytokines, which leads to a chronic inflammatory process which: a) violates the transmission of the insulin signal and, b) impairs mitochondrial function, which violates the homeostasis of glucose. In particular, secreted by fat cells, IL-6 and TNF-alpha weight of IR, and secreted angiotensin II, increases blood pressure and promotes atherosclerosis.
Violation of adaptive mechanisms.
In the cell from fatty acids, not used for β-oxidation, the phospholipids are synthesized first, and then triglycerides, which accumulate in the cytoplasm. Intracellular triglycerides in non-fatty tissues contain mainly palmitic acid. Of palmitic acid is synthesized sphingomyelin, which is a major component of membrane rafts, involved in regulating the activity of membrane receptors.
The synthesis of sphingomyelin, depending on the content in the cell palmitin howling acid, by way of "palmitic acid → ceramide → sphingomyelin". It is the way of synthesis of ceramide from palmitic acid leads to oxidative apoptosis. Ceramide is an inducer of apoptosis as in the oxidative pathway (ceramide inhibits complex III of the etc, causing an enhanced generation of oxidants), and without the involvement of mitochondria.
The accumulation of triglycerides in cardiomyocytes is associated with a reduction of cardiolipin synthesis and the change in the respiratory function of mitochondria, since cytochrome C oxidase of the complex IV of ETS is associated with cardiolipin. Changing the structure of the mitochondrial membrane leads to the release of cytochrome C and apoptosis without the involvement of oxidants. Thus, the accumulation of palmitic acid in the cells of non-fat tissue leads to increased synthesis of ceramide and the decreased synthesis of cardiolipin that induces apoptosis, and altering the activity of receptors.
In this regard, the accumulation in the cells of triglycerides (triglycerides not induce apoptosis) is considered as the body's attempt to avoid the effect of lipotoxicity.
Sphingomyelin, and palmitic acid exhibit a high affinity for cholesterol. Increase in content of sphingomyelin, and palmitic acid in the membrane may explain associated with age accumulation in membranes of cholesterol and sensitivity of insulin receptor.

The insulin receptor is associated with membrane rafts, and changes in the composition of rafts affects its sensitivity. Accumulation of triglycerides in non-fat tissues and associated reduced sensitivity of the insulin receptor leads to insulin resistance and hyperglycemia, i.e. increased content of glucose in the blood. Receptor to insulin is tirozinkinazy.
Autophosphorylation are activated through various ways, in particular, the path PI-3-K (phosphoinositol-3-Kenaz), which is the transport of glucose inside the cell, as it comes in its active operating state of the glucose Transporter GLUT4.
Due to active lipolysis of free fatty acids (FFA) and proinflammatory cytokines, they affect substrates of the insulin receptor and thereby blocks the path PI-3-K, resulting in blocked effects this way on the glucose metabolism, and glucose cannot enter the cell. In this way develops insulin resistance, i.e. an excessive amount of visceral adipose tissue inhibits insulin signal and causes insulin receptors become insensitive to insulin and its biological role is perverted.
Depending on individual sensitivity and genetics, insulin resistance can develop in different tissues. The abundance of FLC mediates the progression of insulin resistance in many tissues — muscle, including myocardial, hepatic, adipose and endothelial cells, promotes the progression of ischemic changes in the myocardium, including changes related to violation of the beta-oxidation of FFA to the myocardium.
Step five: hepatic and muscle insulin resistance.
In conditions of high intake of food rich in fats and carbohydrates, stimulates insulin secretion, which in turn activates lipogenesis and deposition of SFA in the adipose tissue. However, there are genetically-determined limit the ability to accumulate lipids, so when the amount of adipose tissue reaches its maximum, the excess FLC begins to flow into the liver and muscles.
The abundance of FLC is accompanied by accumulation of triglycerides in parenchymal cells of many tissues, namely skeletal and cardiac myocytes and hepatocytes, which leads to their damage and chronic dysfunction.
As a result, the liver under conditions of insulin resistance begins to actively synthesize fatty acids, triglycerides, accelerated lipolysis, but in adipose tissue. In addition, in the liver occur all those processes that lead the patient with visceral obesity to diabetes: stimulates gluconeogenesis and inhibiting glycolysis and glycogen synthesis.

Clarified a rather complex plan, which reflects that insulin resistance, or insulin resistance, leads to the fact that the liver is becoming overwhelmed fatty acids.
This is due to the fact that the synthesized active fatty acid in the liver, decreased oxidation of fatty acids, are actively fatty acids to the liver from visceral adipose tissue and, in addition, the fat in the composition of chylomicrons is supplied to us in the liver, also overloads the liver the free fatty acids.
These processes lead to the fact that the liver is unable to metabolize by β-oxidation of FFA-activated lipid peroxidation, resulting in large quantities are produced by reactive oxygen species, oxidative stress occurs and that these factors lead to the phosphorylation of a substrate of the insulin receptor, what we talked about on the previous slide, thereby again starts the insulin resistance, i.e. a kind of vicious circle, and to determine the patient that is primary is difficult.
It has been shown that macrophages of visceral adipose tissue have anti-inflammatory activity. Also in adipose tissue was found and CD 8 + T-lymphocytes that actively secrete cytokines provospalitelna and, thus, liver steatosis can already move to the next stage with the development in patients of Nash and other consequences, which leads to NAFLD.

Muscle insulin resistance.
Soon after the liver, fat begins to accumulate in the muscles. Characteristic IR metabolic pathology is accumulation around muscle fibrils triglycerides. However, the accumulation of triglycerides within skeletal muscle, as expected, is not the direct cause of the development of type 2 diabetes, but similar. may be marker of lipid intermediates. such as acetyl COA, ceramides and diacylglycerol
According to a recent study, impaired transmission of the insulin signal is associated mostly with abnormal FFA metabolism in cells of skeletal muscle, "not coping" with their recycling, when FLC in excess. Indeed, local accumulation within skeletal muscle FFA metabolites such as ceramides, diglycerol or acyl-COA leads to impaired transmission of the insulin signal and, thus, to disruption of glucose transport.

Step six. The vicious cycle of insulin resistance.
Insulin resistance caused by high FFA level, further increases the concentration of FLC in plasma. It had been revealed that insulin-resistant fat cells secrete elevated levels of FLC. It is, in fact, allows to consider elevated levels of FLC marker of IR.
Indeed, when IRA level of FFA in hepatocytes is increased, because, they:
1) increased de novo lipogenesis,
2) eterificare FLC exceeds their oxidation,
3) esterified LCD stored as triglycerides or are directed to the synthesis of X-VLDL (rich in triglycerides),
4) adjustable insulin decreases mobilization of triglycerides.
Insulin-resistant adipocytes is intensively contained in them break down triglycerides and release of FFA formed them into the bloodstream (as in obesity, and without it). The flow of FFA from fat cells is increased and, moreover, FFA also come from X-VLDL and chylomicrons from plasma and blood were being used in other bodies, and partly back to the liver where it is again converted to triglycerides. There is a "pumping" of the liver, FFA, and triglycerides. It has the most serious consequences.

Step seven. Acceleration of atherosclerosis.
Elevated levels of FFA leads to dyslipidemia and atherogenesis
How insulin resistance leads to dyslipidemia Increase in liver triglycerides stimulates the formation of APO and X-VLDL,
This is how it happens.
1) From the liver to high levels of X-VLDL secreted into the plasma, where due to lipolysis from X-VLDL are formed of FLC and vysokoyarusnye remnant (residual) particles of lipoproteins rich in triglycerides.
2) From plasma FFA and remnant particles are again absorbed by the liver, which further increases the level of FFA in the hepatocytes and further stimulates the synthesis of X-VLDL.
3) In the liver, with a high level X-VLDL normal level of protein CETP (cholesteryl ester transfer protein) is a Transporter of cholesterol ester, triglycerides of X-VLDL are transferred in the X-HDL, and cholesterol from X-HDL is transferred in the X-VLDL. In the end, consist of: a) very cholesterol-rich atherogenic particles remnant X-VLDL, and b) X-HDL, containing a lot of triglycerides and a little cholesterol.
4) These particles X-HDL lose triglycerides (hepatic lipase) and their main apolipoprotein, APO A1. In the end, the level antiaterogennoe X-HDL is lowered.
5) At high levels of X-VLDL (rich in triglycerides), CETP transfers triglycerides from X-VLDL, S-LDL, and cholesterol from X-X in LDL-VLDL.
6) Rich in triglycerides, S-LDL because of the activity of hepatic or lipoprotein lipase, lose triglycerides, decrease in size and become highly atherogenic small dense particles X-LDL.
Thus, elevated levels of FFA reduce the level of "antiatherogenic" X-HDL, the formation of highly atherogenic small dense particles X -, LDL-cholesterol and increased plasma triglyceride levels.

But there is another way in which high levels of FFA induce atherogenesis. This path is more direct and short. When IL increased the level of FFA in the mitochondria causes macrovascular endothelial cells surgentes of reactive oxygen species, leading to oxidation of h-LDL and modification of X-HDL. It induces an inflammatory process in the vessel walls, the formation and accumulation of plaque and results in ischemia.published
Sources.
1. The Publication Of V. V. Velkova
2. Vladimir Velkov. Laboratory diagnosis. – 2009. Vol. 3, No. 49. – P 55 to 71.
3. Velkov V. V. the Free fatty acids as a risk factor for insulin resistance and ischemia: prospects for risk assessment and diagnosis
4. L. I. Danilova Phenomenon of insulin resistance in clinical practice: mechanisms of formation and possibilities of correction
5. Tereshina E. V. the Role of fatty acids in the development of age-related oxidative stress. Advances in gerontology, 2007. Vol. 20, No. 1
6. "Endocrinology and metabolism", F. Felig, D. Baxter
Author: Andrey Blueskin
Psychosomatics diseases of the neck, Through the throat we "swallow" the reality
We shouldn'T treat
Source: www.beloveshkin.com/2016/01/svobodnye-zhirnye-kisloty-klyuch-k-ponimaniyu-insulinorezistentnosti.html