421
Open method of obtaining graphene from ethylene
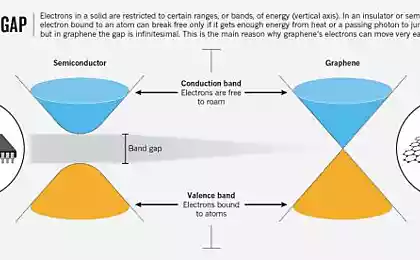
An international team of scientists (USA, Scotland, Germany) have developed a new method of obtaining graphene, the layer of carbon one atom thick. The technology involves the exposure of high temperature on ethylene. This is the simplest alkene contains 2 carbon atoms, which subsequently form a two-dimensional layer of graphene.
Fifty seven million nine hundred seventy one thousand fifty nine
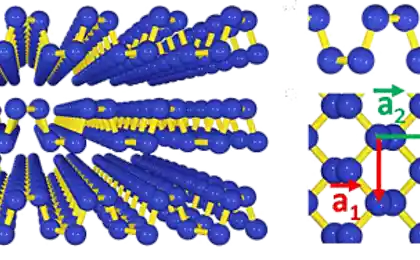
Scientists placed ethylene on a substrate of rhodium catalyst and gradually heated to a temperature of 700 °C. This allowed them to layers of pure graphene. In the previous similar research, the scientists made mistakes in their attempts to obtain graphene from hydrocarbons: the temperature was below, does not comply with the gradation heating. Now managed to establish the optimal combination of conditions for obtaining graphene in this way.
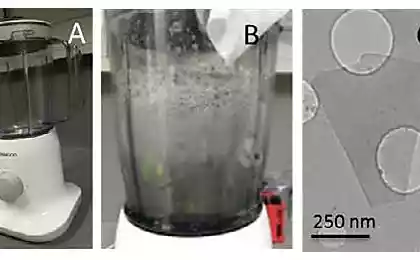
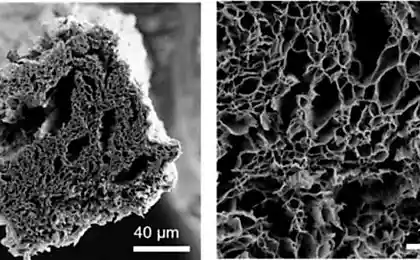
The advantage of this method is that the ethylene is available and cheap. As graphene is composed of carbon, scientists have tried to extract it from the simplest carbon-containing molecules. Many attempts were unsuccessful — instead of graphene was disordered soot. But at some point, the small molecules were able to obtain macroscopic graphene flakes.
Seventy five million fifteen thousand nine hundred thirty eight
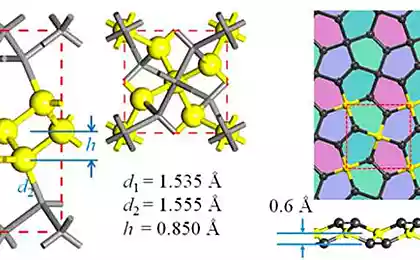
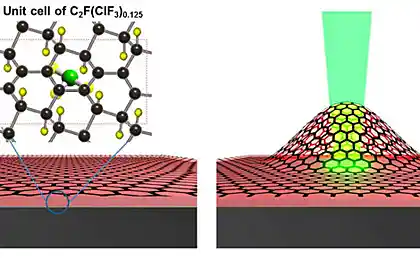
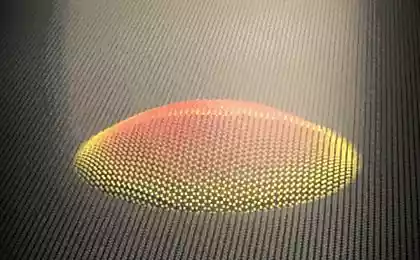
Guided by the theory, the researchers found that during the transition to graphene, the ethylene must go through several stages. During these stages the ethylene loses a hydrogen, and the carbon independently arranged in a honeycomb structure that characterizes graphene. This process was really to recreate by stepwise heating of ethylene. This material, as has been said, was on a substrate of rhodium catalyst, which induced the conversion of ethylene to graphene.
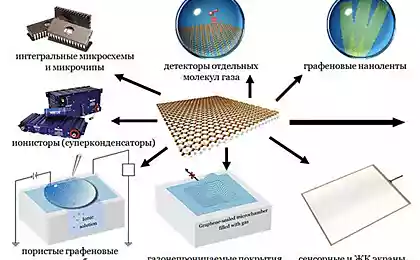
Applications of graphene are more and more, along with this keeps popping up on news about reducing the cost and simplifying the manufacturing process. Recently, scientists have managed to cope with the high combustibility of graphene — one of the reasons for which are still not mass-produced. In addition to industrial methods, the researchers find, and less ambitious, but more effective. So graphene can be obtained in the microwave, and if conditions permit, it is possible to organize the explosion of a mixture of oxygen and ethylene, leading to the loss of graphene. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //hightech.fm/2017/05/05/graphene_ethene
Fifty seven million nine hundred seventy one thousand fifty nine
Scientists placed ethylene on a substrate of rhodium catalyst and gradually heated to a temperature of 700 °C. This allowed them to layers of pure graphene. In the previous similar research, the scientists made mistakes in their attempts to obtain graphene from hydrocarbons: the temperature was below, does not comply with the gradation heating. Now managed to establish the optimal combination of conditions for obtaining graphene in this way.
The advantage of this method is that the ethylene is available and cheap. As graphene is composed of carbon, scientists have tried to extract it from the simplest carbon-containing molecules. Many attempts were unsuccessful — instead of graphene was disordered soot. But at some point, the small molecules were able to obtain macroscopic graphene flakes.
Seventy five million fifteen thousand nine hundred thirty eight
Guided by the theory, the researchers found that during the transition to graphene, the ethylene must go through several stages. During these stages the ethylene loses a hydrogen, and the carbon independently arranged in a honeycomb structure that characterizes graphene. This process was really to recreate by stepwise heating of ethylene. This material, as has been said, was on a substrate of rhodium catalyst, which induced the conversion of ethylene to graphene.
Applications of graphene are more and more, along with this keeps popping up on news about reducing the cost and simplifying the manufacturing process. Recently, scientists have managed to cope with the high combustibility of graphene — one of the reasons for which are still not mass-produced. In addition to industrial methods, the researchers find, and less ambitious, but more effective. So graphene can be obtained in the microwave, and if conditions permit, it is possible to organize the explosion of a mixture of oxygen and ethylene, leading to the loss of graphene. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //hightech.fm/2017/05/05/graphene_ethene