387
Lithium-air fuel cell can be stabilized
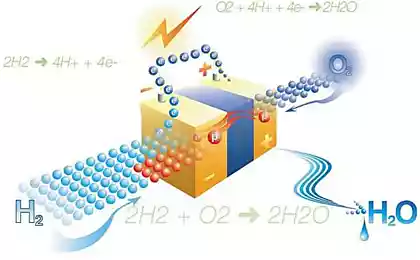
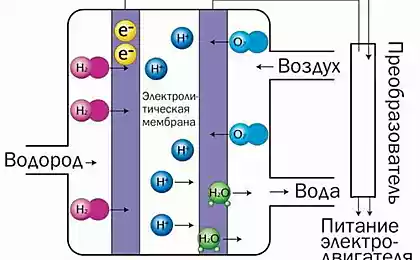
For specific power consumption, such devices benefit in comparison with fossil fuels. But there are major obstacles to their massive deployment. The list includes the inability of normal recharge, the loss of efficiency because of the strong surge and low specific energy consumption.
Thirty two million eight hundred sixty one thousand one hundred ninety four
Such characteristics contributes to 2 types of instability, which focused laboratory Professor Lynden Archer at Cornell University. First, professionals are mainly engaged in the question of the formation of nuclei and growth of dendrites from one electrode to another. The process led to a short circuit and premature failure of the battery. In a new study presented in the Science Advances, the team focused on the deterioration of the throughput. Graduate student, Snehasish Chaudhuri proposed a solution to this problem.
Eighty three million six hundred fifty four thousand two hundred seventy eight
Problems arise when the electrolyte begins to react with the electrodes, forming an insulating compounds that inhibit the transport of ions. Completely abandon the interaction of the components is impossible – it is necessary for battery life. The solution Chaudhuri is an artificial intermediate phase of solid electrolyte (SEI) – the material that protects the electrodes and at the same time contributing to the movement of electrons from one end of the battery to another.
Such structures are formed in all electrochemical cells. Their stability is critical to the work"s graphite anode of lithium-ion batteries," said Archer.
Intermediate phase Chaudhuri based on ionic polymers with bromide. Selectively crepes to the lithium anode, the material forms a thin protective layer, preventing its destruction. SEI decided 3 problems: stop the growth of dendrites, "pacified" the redox processes, reducing stress, and protect the metal without interfering with the movement of ions. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //www.sciencedaily.com/releases/2017/04/170426153813.htm
Thirty two million eight hundred sixty one thousand one hundred ninety four
Such characteristics contributes to 2 types of instability, which focused laboratory Professor Lynden Archer at Cornell University. First, professionals are mainly engaged in the question of the formation of nuclei and growth of dendrites from one electrode to another. The process led to a short circuit and premature failure of the battery. In a new study presented in the Science Advances, the team focused on the deterioration of the throughput. Graduate student, Snehasish Chaudhuri proposed a solution to this problem.
Eighty three million six hundred fifty four thousand two hundred seventy eight
Problems arise when the electrolyte begins to react with the electrodes, forming an insulating compounds that inhibit the transport of ions. Completely abandon the interaction of the components is impossible – it is necessary for battery life. The solution Chaudhuri is an artificial intermediate phase of solid electrolyte (SEI) – the material that protects the electrodes and at the same time contributing to the movement of electrons from one end of the battery to another.
Such structures are formed in all electrochemical cells. Their stability is critical to the work"s graphite anode of lithium-ion batteries," said Archer.
Intermediate phase Chaudhuri based on ionic polymers with bromide. Selectively crepes to the lithium anode, the material forms a thin protective layer, preventing its destruction. SEI decided 3 problems: stop the growth of dendrites, "pacified" the redox processes, reducing stress, and protect the metal without interfering with the movement of ions. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //www.sciencedaily.com/releases/2017/04/170426153813.htm
Green smoothie with spirulina and spinach
Germany has successfully tested the first electric car letowski