546
A fuel cell or an eternal battery
Fuel cell homes with their own hands
Improving every year mobile electronics becoming more affordable and rasprostranenie: PDA, laptops, mobile and digital devices, photo frames etc. All of them are all the time updated with new features, large monitors, wireless connectivity, more powerful processors, while decreasing in size. Technology power, unlike the semiconductor technology, strides are not going.
The existing batteries and accumulators to power the achievements of the industry is not enough, so the question of alternative sources is very serious. Fuel cells are today the most promising direction. The principle of their work odrt was in 1839 William Groom that electricity generated by changing the electrolysis of water.
What are fuel cells?
Video: Documentary film fuel cells for transportation: past, present, future
Fuel cells are interesting to car manufacturers, are interested in them and the creators of the spacecraft. In 1965, they were even tested by America launched the space ship "Gemini-5" and later "Apollo". Millions of dollars are being invested in research of fuel cells and today, when there are problems with environmental pollution, increasing vibratome greenhouse gases produced by the combustion of fossil fuels, whose reserves are also not infinite.
The fuel cell, often called electrochemical generator works the following way. Ninety seven million three hundred four thousand four hundred ten
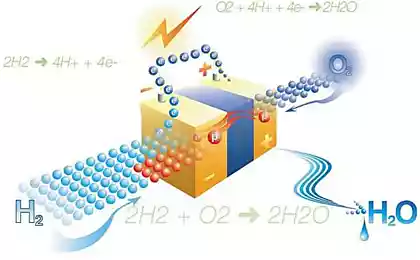
The scheme of operation of the Fuel cell on hydrogen
Being, as the batteries of the galvanic element, but with the difference that stored in the active substances separately. Electrodes they act as. On the negative electrode burns fossil fuels or any substance derived from it, which can be gaseous (hydrogen, for example, and carbon monoxide) or liquid, like alcohols. On the electrode positive, as a rule, reacts with oxygen.
But easy on the principle of action, in reality to realize not just.
Fuel cell with their hands
Unfortunately we have no photos of how it should look like this fuel elecment, we hope for your imagination.
Low-power fuel cell with their hands can be made even in the conditions of a school laboratory. Need to stock up on the old gas mask, a few pieces of plexiglass, with alkali and an aqueous solution of ethyl alcohol (vodka), which will serve to fuel cell "fuel".
Three million three hundred forty six thousand seven hundred eighty two
Stationary power plant on the basis of the chemical fuel cell
First of all, the necessary housing for a fuel cell, to make which is better than Plexiglas, with a minimum thickness of five millimeters. Internal partitions (inside five compartments) can be made a little thinner – 3 cm For gluing plexiglass glue used this composition: one hundred grams of chloroform or dichloroethane dissolve six grams of shavings of Plexiglas (work under the hood).
In the outer wall now you need to drill a hole in which to insert through the rubber stopper drain a glass tube with a diameter of 5-6 centimeters.
Everyone knows that in the periodic table in the lower left corner are the most active metals and metalloids of high activity are given in the table in the upper right corner, i.e. the ability to give electrons increases from top to bottom and from right to left. Members that can under certain conditions manifest itself as a metals or metalloids, are located in the centre of the table.
Now the second and fourth division pours out of a gas mask activated carbon (between the first partition and the second and third and fourth), which will perform the role of electrodes. Through hole coal does not spill, it can be placed in a nylon fabric (women's stockings).
The fuel will circulate in the first chamber, the fifth must be the supplier of oxygen – air. Between the electrodes will be in the electrolyte, and to ensure that he was not able to seep into the air chamber, before filling the fourth chamber of the coal to air, the electrolyte, to impregnate it with a solution of paraffin in gasoline (a ratio of 2 grams of wax in a half Cup of gasoline). On the coal layer need to put a (slightly pushing) copper plates, to which are soldered wires. Through them a current will be discharged from the electrodes.
It remains only to charge the item. That's what the vodka to dilute with water in 1:1. Then carefully add three hundred-three hundred and fifty grams of potassium hydroxide. For the electrolyte 200 grams of water is dissolved 70 grams of potassium hydroxide. Fuel cell ready to test. Now you want to pour into the first chamber – the fuel, and the third electrolyte. Attached to the electrodes, the voltmeter should show from 07 volt to 0.9. To ensure continuous operation of the element, you need to take the spent fuel (drain the Cup) and add new (via rubber tube). The feed rate is regulated by the compression of the tube. It looks in laboratory conditions, the fuel cell, whose power, clear small.
So the power was greater, scientists have been working on this issue. On the active steel development are methanol and ethanol fuel cells. But, unfortunately, on the practice of their way.
Why the fuel cell is selected as the alternative power source
Twenty four million five hundred ten thousand nine hundred twenty six
Working model toy-electric car hydrogen fuel cell
An alternative source of supply of the selected fuel cell, since the end product of combustion of hydrogen in it is water. The problem is finding cheap and efficient method of producing hydrogen. Huge funds invested in the development of hydrogen generators and fuel cells, cannot fail to bear fruit, so a technological breakthrough and their real use in daily life, only a matter of time.
Today the monsters of the automotive industry: "General motors", "Honda", "Driller Kaisler", "Ballard", showing the buses and cars that run on fuel cells, a capacity up to 50kW. But, the problems associated with their safety, reliability, cost — not yet decided. As mentioned, unlike conventional power sources – batteries, in this case, the oxidizer and fuel are served outside, but the fuel cell only is the mediator of the reaction occurring in the fuel combustion and the transformation into electricity released energy. Flows "burning" only in the case if the item gives the current in the load, like a diesel generator but without the generator and diesel, but without noise, smoke and overheating. In this case, the efficiency is much higher, since there are no intermediate mechanisms.
There are high hopes for the application of nanotechnology and nanomaterials, which will help to miniaturise fuel cells to increase their power. There were messages that created a super-efficient catalysts, and the design of fuel cells without membranes. Them together with the oxidant is supplied to the element of fuel (methane, for example). Interesting solutions, where as oxidant is used, the oxygen dissolved in the water of the air, and as fuel – the organic impurities accumulating in contaminated waters. This so-called biofuel elements.
Fuel cells, according to forecasts of specialists, the mass market may go in the coming years. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: motocarrello.ru/jelektrotehnologii/1242-toplivnyj-jelement-svoimi-rukami.html
Improving every year mobile electronics becoming more affordable and rasprostranenie: PDA, laptops, mobile and digital devices, photo frames etc. All of them are all the time updated with new features, large monitors, wireless connectivity, more powerful processors, while decreasing in size. Technology power, unlike the semiconductor technology, strides are not going.
The existing batteries and accumulators to power the achievements of the industry is not enough, so the question of alternative sources is very serious. Fuel cells are today the most promising direction. The principle of their work odrt was in 1839 William Groom that electricity generated by changing the electrolysis of water.
What are fuel cells?
Video: Documentary film fuel cells for transportation: past, present, future
Fuel cells are interesting to car manufacturers, are interested in them and the creators of the spacecraft. In 1965, they were even tested by America launched the space ship "Gemini-5" and later "Apollo". Millions of dollars are being invested in research of fuel cells and today, when there are problems with environmental pollution, increasing vibratome greenhouse gases produced by the combustion of fossil fuels, whose reserves are also not infinite.
The fuel cell, often called electrochemical generator works the following way. Ninety seven million three hundred four thousand four hundred ten
The scheme of operation of the Fuel cell on hydrogen
Being, as the batteries of the galvanic element, but with the difference that stored in the active substances separately. Electrodes they act as. On the negative electrode burns fossil fuels or any substance derived from it, which can be gaseous (hydrogen, for example, and carbon monoxide) or liquid, like alcohols. On the electrode positive, as a rule, reacts with oxygen.
But easy on the principle of action, in reality to realize not just.
Fuel cell with their hands
Unfortunately we have no photos of how it should look like this fuel elecment, we hope for your imagination.
Low-power fuel cell with their hands can be made even in the conditions of a school laboratory. Need to stock up on the old gas mask, a few pieces of plexiglass, with alkali and an aqueous solution of ethyl alcohol (vodka), which will serve to fuel cell "fuel".
Three million three hundred forty six thousand seven hundred eighty two
Stationary power plant on the basis of the chemical fuel cell
First of all, the necessary housing for a fuel cell, to make which is better than Plexiglas, with a minimum thickness of five millimeters. Internal partitions (inside five compartments) can be made a little thinner – 3 cm For gluing plexiglass glue used this composition: one hundred grams of chloroform or dichloroethane dissolve six grams of shavings of Plexiglas (work under the hood).
In the outer wall now you need to drill a hole in which to insert through the rubber stopper drain a glass tube with a diameter of 5-6 centimeters.
Everyone knows that in the periodic table in the lower left corner are the most active metals and metalloids of high activity are given in the table in the upper right corner, i.e. the ability to give electrons increases from top to bottom and from right to left. Members that can under certain conditions manifest itself as a metals or metalloids, are located in the centre of the table.
Now the second and fourth division pours out of a gas mask activated carbon (between the first partition and the second and third and fourth), which will perform the role of electrodes. Through hole coal does not spill, it can be placed in a nylon fabric (women's stockings).
The fuel will circulate in the first chamber, the fifth must be the supplier of oxygen – air. Between the electrodes will be in the electrolyte, and to ensure that he was not able to seep into the air chamber, before filling the fourth chamber of the coal to air, the electrolyte, to impregnate it with a solution of paraffin in gasoline (a ratio of 2 grams of wax in a half Cup of gasoline). On the coal layer need to put a (slightly pushing) copper plates, to which are soldered wires. Through them a current will be discharged from the electrodes.
It remains only to charge the item. That's what the vodka to dilute with water in 1:1. Then carefully add three hundred-three hundred and fifty grams of potassium hydroxide. For the electrolyte 200 grams of water is dissolved 70 grams of potassium hydroxide. Fuel cell ready to test. Now you want to pour into the first chamber – the fuel, and the third electrolyte. Attached to the electrodes, the voltmeter should show from 07 volt to 0.9. To ensure continuous operation of the element, you need to take the spent fuel (drain the Cup) and add new (via rubber tube). The feed rate is regulated by the compression of the tube. It looks in laboratory conditions, the fuel cell, whose power, clear small.
So the power was greater, scientists have been working on this issue. On the active steel development are methanol and ethanol fuel cells. But, unfortunately, on the practice of their way.
Why the fuel cell is selected as the alternative power source
Twenty four million five hundred ten thousand nine hundred twenty six
Working model toy-electric car hydrogen fuel cell
An alternative source of supply of the selected fuel cell, since the end product of combustion of hydrogen in it is water. The problem is finding cheap and efficient method of producing hydrogen. Huge funds invested in the development of hydrogen generators and fuel cells, cannot fail to bear fruit, so a technological breakthrough and their real use in daily life, only a matter of time.
Today the monsters of the automotive industry: "General motors", "Honda", "Driller Kaisler", "Ballard", showing the buses and cars that run on fuel cells, a capacity up to 50kW. But, the problems associated with their safety, reliability, cost — not yet decided. As mentioned, unlike conventional power sources – batteries, in this case, the oxidizer and fuel are served outside, but the fuel cell only is the mediator of the reaction occurring in the fuel combustion and the transformation into electricity released energy. Flows "burning" only in the case if the item gives the current in the load, like a diesel generator but without the generator and diesel, but without noise, smoke and overheating. In this case, the efficiency is much higher, since there are no intermediate mechanisms.
There are high hopes for the application of nanotechnology and nanomaterials, which will help to miniaturise fuel cells to increase their power. There were messages that created a super-efficient catalysts, and the design of fuel cells without membranes. Them together with the oxidant is supplied to the element of fuel (methane, for example). Interesting solutions, where as oxidant is used, the oxygen dissolved in the water of the air, and as fuel – the organic impurities accumulating in contaminated waters. This so-called biofuel elements.
Fuel cells, according to forecasts of specialists, the mass market may go in the coming years. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: motocarrello.ru/jelektrotehnologii/1242-toplivnyj-jelement-svoimi-rukami.html
Put an oxygen mask on yourself and then on your baby.
The less you know the better you sleep, or learn to filter