467
New more effective solar-fuel cell with nanowires of gallium phosphide
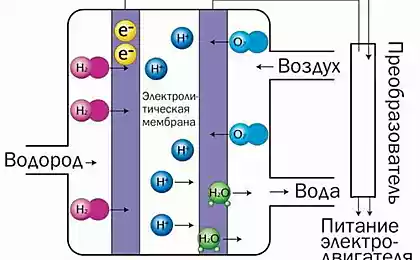
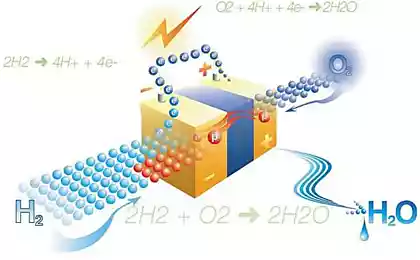
One of the most promising forms of artificial photosynthesis is to use solar energy to split water to produce oxygen and hydrogen which can be stored and used as clean fuel. And one of the most promising semiconductor materials for such a task is gallium phosphide (GaP), which not only can convert sunlight into electricity, but also to split water. Unfortunately, this material is quite expensive, but researchers from the Technological University of Eindhoven (TU/e) and the FOM Foundation recently announced the development of prototype solar-fuel cell in which gallium phosphide is used in a processed form. This allowed not only 10 thousand times to reduce the consumption of precious material, but also to increase the hydrogen yield by 10 times. 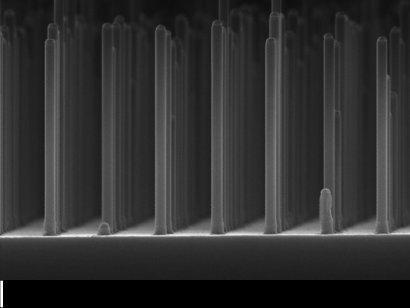
It is worth noting that when you connect a standard silicon solar cell to the fuel element, the efficiency of converting water into hydrogen and oxygen reaches 15 percent. Of course, solar and fuel cells based on gallium phosphide has a much lower efficiency photoelectrochemical reaction, but its obvious advantage is compactness. Well, the new nanowires of gallium phosphide allowed to increase the yield of hydrogen to 2.9 percent, which is 10 times higher than when using plates of the same material.
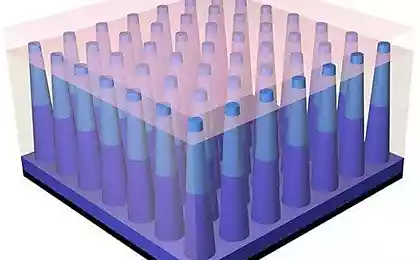
The researchers grew nanowires in structured, orderly arrays. Each nanowire has dimensions of 500 nm in length and 90 nm thick. Thanks to this optimized geometrically correct form of nanowires, scientists have been able to significantly increase the surface area for more efficient absorption of sunlight, and in a range of wavelengths. In addition, these arrays of nanowires can be installed on a substrate made of a flexible polymer.
However, the researchers acknowledge that the obtained hybrid solar-fuel cell is still relatively low efficiency. But according to them, this element has great potential to increase the efficiency, and they plan to continue to work to improve the prototype.
P. S. And remember, just changing your mind — together we change the world! ©
Source: www.cheburek.net/energiya-solnca/novyj-bolee-effektivnyj-solnechno-toplivnyj-element-s-nanoprovodami-iz-fosfida-galliya.html

It is worth noting that when you connect a standard silicon solar cell to the fuel element, the efficiency of converting water into hydrogen and oxygen reaches 15 percent. Of course, solar and fuel cells based on gallium phosphide has a much lower efficiency photoelectrochemical reaction, but its obvious advantage is compactness. Well, the new nanowires of gallium phosphide allowed to increase the yield of hydrogen to 2.9 percent, which is 10 times higher than when using plates of the same material.
The researchers grew nanowires in structured, orderly arrays. Each nanowire has dimensions of 500 nm in length and 90 nm thick. Thanks to this optimized geometrically correct form of nanowires, scientists have been able to significantly increase the surface area for more efficient absorption of sunlight, and in a range of wavelengths. In addition, these arrays of nanowires can be installed on a substrate made of a flexible polymer.
However, the researchers acknowledge that the obtained hybrid solar-fuel cell is still relatively low efficiency. But according to them, this element has great potential to increase the efficiency, and they plan to continue to work to improve the prototype.
P. S. And remember, just changing your mind — together we change the world! ©
Source: www.cheburek.net/energiya-solnca/novyj-bolee-effektivnyj-solnechno-toplivnyj-element-s-nanoprovodami-iz-fosfida-galliya.html