903
Fuel cells: types and working principle
A fuel cell is a device that efficiently generates direct current and heat from hydrogen rich fuels through an electrochemical reaction.
A fuel cell is similar to battery in that it produces direct current by chemical reaction. Again, like the battery, fuel elementvalue anode, cathode and electrolyte. However, unlike batteries, fuel cells can not accumulate electric energy, do not discharge and do not require electricity for re-charging. Fuel cells can continuously generate electricity as long as they have fuel and air. The correct term to describe the working of a fuel cell is a system of elements so as to complete the work required by the presence of some auxiliary systems.
Unlike other power generators such as internal combustion engines or turbines operating on gas, coal, oil etc., fuel cells do not burn fuel. This means no noisy high-pressure rotors, loud exhaust noise and vibrations. Fuel cells produce electricity through a silent electrochemical reaction. Another feature of fuel cells is that they convert the chemical energy of a fuel directly into electricity, heat and water.
Fuel cells are highly efficient and do not produce large amounts of greenhouse gases such as carbon dioxide, methane and nitrous oxide. The only product release from the fuel elements are water in the form of steam and small quantities of carbon dioxide, which is generally not allocated, if the fuel is pure hydrogen. The fuel elements are collected into assemblies, and then in a separate function modules.
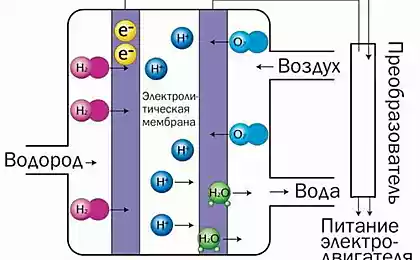
The working principle of fuel cellsFuel cells produce electricity and heat due to the ongoing electrochemical reactions with the electrolyte, the cathode and anode.
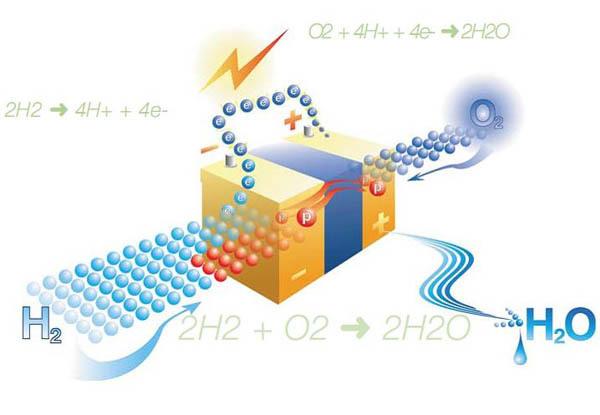
The anode and cathode separated by electrolyte, conducting protons. After the hydrogen goes to the anode, and oxygen to the cathode, starts a chemical reaction, which generates electric current, heat and water. On the catalyst of the anode, molecular hydrogen dissociates and loses electrons. Hydrogen ions (protons) are conducted through the electrolyte to the cathode, while the electrons are ignored by the electrolyte and pass through the external circuit, creating a DC current that can be used to power the equipment. On the catalyst of the cathode, the oxygen molecule joins with an electron (which is supplied from an external communications) and came with a proton, and forms water, which is the only product of the reaction (in the form of steam and/or fluid).
The following is the response:
The reaction at the anode: 2H2 => 4H+ + 4e-
The reaction at the cathode: O2 + 4H+ + 4e- => 2H2O
The General reaction of the elements: 2H2 + O2 => 2H2O
Types of fuel cells
Like the existence of various types of internal combustion engines, there are different types of fuel cells – selection of suitable type of fuel cells depends on its application.Fuel cells are divided into high temperature and low temperature. Low-temperature fuel cells require the fuel is relatively pure hydrogen.
This often means that require fuel processing to convert the primary fuel (such as natural gas) into pure hydrogen. This process consumes extra energy and requires special equipment. High temperature fuel cells do not need this additional procedure, as they can implement "transformation" of the fuel at elevated temperatures, which means no need of investing money in hydrogen infrastructure.
Fuel cells molten carbonate (MCFC).
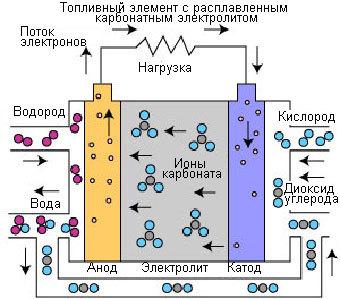
Fuel cells having molten carbonate electrolyte are high-temperature fuel cells. High operating temperature allows direct use of natural gas without the fuel processor and fuel gas with low calorific value fuel production processes and from other sources. This process was developed in the mid-1960s, Since that time has improved the technology of production, performance and reliability.
The work of the MCFC is different from other fuel cells. These elements use an electrolyte composed of a molten carbonate salt mixture. Currently, two types of compounds: lithium carbonate and potassium carbonate or lithium carbonate and sodium carbonate. For melting the carbonate salts and achieve high degree of mobility of ions in the electrolyte, the fuel cell with molten carbonate electrolyte occurs at high temperatures (650°C). Efficiency varies in the range of 60-80%.
When heated to a temperature of 650°C, the salt becomes a conductor of carbonate ions (CO32-). These ions pass from the cathode to the anode, where there is a combining with hydrogen to form water, carbon dioxide and free electrons. These electrons are directed through the external circuit back to the cathode, this will generate an electric current, and as a byproduct – heat.
The reaction on the anode: CO32- + H2 => H2O + CO2 + 2e-
The reaction at the cathode: CO2 + 1/2O2 + 2e- => CO32-
The General reaction of the elements: H2(g) + 1/2O2(g) + CO2(cathode) => H2O(g) + CO2(anode)
High operating temperature fuel cells with molten carbonate electrolyte have certain advantages. At high temperatures, internal reforming of natural gas, which eliminates the need for a fuel processor. In addition, the advantages include the possibility of using the standard materials of construction such as stainless steel sheets and Nickel catalyst on the electrodes. Product heat can be used to generate high pressure steam for various industrial and commercial purposes.
The high reaction temperature in the electrolyte also have their advantages. The use of high temperature requires significant time to achieve optimal operating conditions, the system responds more slowly to changes in energy consumption. These characteristics allow for installation on fuel cells with molten carbonate electrolyte under conditions of constant power. High temperatures prevent damage to the fuel cell carbon monoxide "poisoning", etc.
Fuel cells with molten carbonate electrolyte suitable for use in large stationary installations. Industrial produced heat and power installation with an output electrical power of 2.8 MW. Developed installation with output power up to 100 MW.
Fuel cells based on phosphoric acid (PCTA).
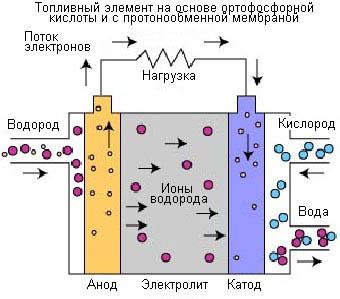
Fuel cells based on phosphoric (orthophosphoric) acid were the first fuel cells for commercial use. This process was developed in the mid-1960s, tests were conducted in the 1970s, Since that time has been increased stability, performance and reduced cost.
Fuel cells based on phosphoric (orthophosphoric) acid is used an electrolyte based on phosphoric acid (H3PO4) with a concentration of up to 100%. The ionic conductivity of phosphoric acid is low at low temperatures, for this reason, these fuel cells are used at temperatures of up to 150-220°C.
Carrier charge in the fuel cells of this type is the hydrogen (H+, proton). A similar process takes place in fuel cells with a membrane of exchange of protons (MOPTA), in which hydrogen supplied to the anode is split into protons and electrons. The protons pass through the electrolyte and combine with oxygen derived from air at the cathode to form water. The electrons are directed through the external circuit, this will generate an electric current. Below are reactions, which generate electric current and heat.
The reaction at the anode: 2H2 => 4H+ + 4e-
The reaction at the cathode: O2(g) + 4H+ + 4e- => 2H2O
The General reaction of the elements: 2H2 + O2 => 2H2O
Efficiency of fuel cells based on phosphoric (orthophosphoric) acid is more than 40% when generating electrical energy. The combined production of heat and electricity, total efficiency is about 85%. In addition, given the operating temperature, waste heat can be used for heating water and generating steam at atmospheric pressure.
High performance of thermal power plants on fuel cells based on phosphoric (orthophosphoric acid) for the combined production of heat and electric energy is one of the advantages of this type of fuel cells. The unit is a carbon monoxide concentration of about 1.5%, significantly expands the choice of fuel. In addition, CO2 does not affect the electrolyte and the fuel cell operation, the type of the elements works with reformirovaniyu natural fuels. Simple design, low degree of volatility of the electrolyte and high stability are the advantages of this type of fuel cells.
Industrial produced heat and power installation with an output electrical power up to 400 kW. Installation of 11 MW tested. Developed installation with output power up to 100 MW.
Fuel cells with a membrane of exchange of protons (MOPTA)
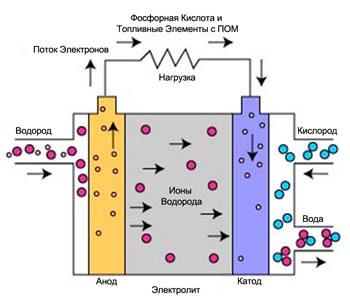
Fuel cells with a membrane of exchange of protons are considered the best type of fuel cells to generate power for vehicles capable of replacing petrol and diesel internal combustion engines. These fuel cells were first used by NASA to program "Gemini". Currently develop and demonstrate installation on MOPTA power from 1 watt to 2 kW.
The electrolyte in these fuel cells use solid polymer membrane (thin plastic film). When saturated with water this polymer transmits protons but does not conduct electrons.
The fuel is hydrogen, and the carrier charge – the hydrogen ion (proton). At the anode, the hydrogen molecule splits into a hydrogen ion (proton) and electrons. The hydrogen ions pass through the electrolyte to the cathode, while the electrons move around the outer circle and produce electrical energy. The oxygen is taken from air, is fed to the cathode and combines with the electrons and hydrogen ions to form water. At the electrodes the following reactions occur:
The reaction at the anode: 2H2 + 4OH- => 4H2O + 4e-
The reaction at the cathode: O2 + 2H2O + 4e- => 4OH-
The General reaction of the elements: 2H2 + O2 => 2H2O
Compared to other types of fuel cells, fuel cells with a membrane of exchange of protons produce more energy in a given volume or weight of fuel cell. This feature allows them to be compact and light. Besides, working temperature less than 100°C, which allows you to quickly start operation. These characteristics, as well as the ability to quickly change the output energy are just some of the features that make these fuel cells are the first candidate for use in vehicles.
Another advantage is that the electrolyte acts as a solid rather than a liquid substance. To keep the gases at both the anode and cathode is easier with the use of a solid electrolyte, and therefore, these fuel cells cheaper to produce. Compared to other electrolytes, the application of solid electrolyte does not occur such difficulties as the orientation, there is less of a problem due to corrosion, which leads to greater durability of the item and its components.
Solid oxide fuel cells (SOFC)
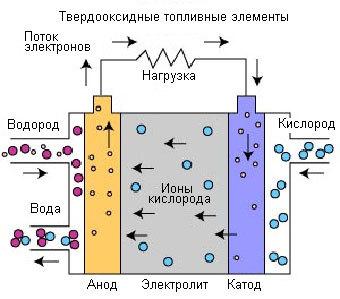
Solid oxide fuel cells are fuel cells with the highest operating temperature. Operating temperature can range from 600°C to 1000°C, which allows you to use different types of fuel without special pre-treatment. To work with such high temperatures used, the electrolyte is a thin solid metal oxide on a ceramic base, often an alloy of yttrium and zirconium, which is a conductor of oxygen ions (O2-). The technology of using solid oxide fuel cells developed since the late 1950s, and has two configurations: planar and tubular.
The solid electrolyte provides a sealed transfer of gas from one electrode to another, while liquid electrolytes are located in the porous substrate. Carrier charge in the fuel cells of this type is the oxygen ion (O2-). At the cathode, splitting the oxygen molecules from the air into oxygen ion and four electrons. The oxygen ions pass through the electrolyte and combine with hydrogen, this produces four free electrons. The electrons are directed through the external circuit, this will generate an electric current and waste heat.
The reaction at the anode: 2H2 + 2O2- => 2H2O + 4e-
The reaction at the cathode: O2 + 4e- => 2O2-
The General reaction of the elements: 2H2 + O2 => 2H2O
Efficiency of produced electric energy is the highest of all fuel cells is about 60%. In addition, the high operating temperatures allow for combined production of thermal and electric energy to generate high pressure steam. The combination of high temperature fuel cell with a turbine allows you to create hybrid fuel cell to increase the efficiency of generating electrical energy up to 70%.
Solid oxide fuel cells operate at very high temperatures (600°C–1000°C), which requires considerable time to achieve optimal operating conditions, the system responds more slowly to changes in energy consumption. At such high operating temperatures do not require the Converter to recover hydrogen from fuel thermal power plant to operate with a relatively impure fuels obtained through gasification of coal or exhaust, etc. Also, this fuel cell is particularly suited to high power, including industrial and large Central power plants. Industrial modules are available with output electric power of 100 kW.
Fuel cells with direct oxidation of methanol (PAMTA)
The technology of using fuel cells with direct oxidation of methanol is undergoing a period of active development. She has successfully established itself in the field of supply mobile phones, laptops, and also for creating portable power sources. which is the aim of future application of these elements.
Device fuel cell with direct oxidation of methanol are similar to fuel cells with a membrane of exchange of protons (MOPTA), i.e. in the electrolyte is a polymer and the charge carrier is the hydrogen ion (proton). However, liquid methanol (CH3OH) is oxidized in the presence of water at the anode to CO2, hydrogen ions and electrons that are directed through the external circuit, this will generate an electric current. The hydrogen ions pass through the electrolyte and react with oxygen from air and electrons supplied from external circuit to form water at the anode.
The reaction at the anode: CH3OH + H2O => CO2 + 6H+ + 6e-
The reaction at the cathode: 3/2O2 + 6H+ + 6e- => 3H2O
A common reaction of the element: CH3OH + 3/2O2 => CO2 + 2H2O
The development of these fuel cells was developed in the early 1990s, After the creation of improved catalysts and, thanks to another recent innovation has been increased power density and efficiency of up to 40%.
Tests were carried out in a temperature range of 50-120°C. Due to the low operating temperatures and no need to use Converter, fuel cells with direct oxidation of methanol is the best candidate for applications as in mobile phones and other consumer goods, and motor vehicles. The advantage of this type of fuel cells are small in size, due to the use of liquid fuel, and no need to use Converter.
Alkaline fuel cells (MTA)
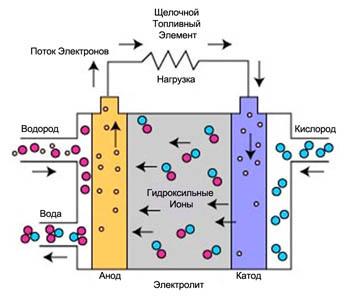
Alkaline fuel cells (MTA) is one of the most proven technologies in use since the mid 1960s, the Agency NASA programs Apollo and the space Shuttle. Aboard these space ships, fuel cells produce electrical energy and drinking water. Alkaline fuel cells are one of the most effective elements used for the generation of electricity, the generation efficiency reaches 70%.
In alkaline fuel cells use an electrolyte that is an aqueous solution of potassium hydroxide contained in a porous stabilized matrix. The concentration of potassium hydroxide can vary depending on the operating temperature of the fuel cell, the range of which varies from 65°C to 220°C. the Carrier charge in STE is the hydroxyl ion (OH-), moving from the cathode to the anode, where it reacts with hydrogen to produce water and electrons. Water obtained on the anode, moves back to the cathode, there again generating hydroxyl ions. As a result of a number of reactions taking place in a fuel cell, produces electricity and, as byproduct, heat:
The reaction at the anode: 2H2 + 4OH- => 4H2O + 4e-
The reaction at the cathode: O2 + 2H2O + 4e- => 4OH-
Overall reaction: 2H2 + O2 => 2H2O
The advantage of STA is that these fuel cells are the cheapest to manufacture because the catalyst required on the electrodes can be any of the substances, cheaper than those used as catalysts for other fuel cells. In addition, STA work at relatively low temperatures and are among the most efficient fuel cells — such features may therefore contribute to the acceleration of generation of power and high fuel efficiency.
One of the characteristic features of STA – high sensitivity to CO2 that can be contained in the fuel or air. CO2 reacts with the electrolyte, poisons it rapidly, and severely reduces the efficiency of the fuel cell. Therefore, the use of STA is limited to closed spaces, such as space and underwater vehicles, they must operate on pure hydrogen and oxygen. Moreover, molecules like CO, H2O and CH4, which are safe for other fuel cells, and some of them even are fuels that are harmful to STA.
Polymer electrolyte fuel cells (PATE)
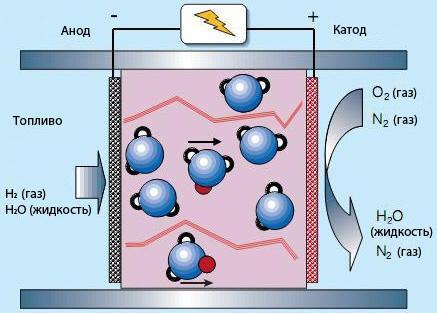
In the case of a polymer electrolyte fuel cell polymer membrane comprises polymer fibers with water areas where there is conductivity of water ions H2O+ (proton, red) attaches itself to the water molecule. Water molecules are a problem because of slow ion exchange. Therefore, it requires a high concentration of water in the fuel, and exhaust electrodes, which limits the working temperature of 100°C.
Tverdokopchenye fuel cells (TCTA)
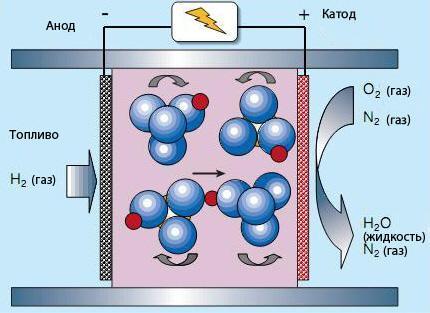
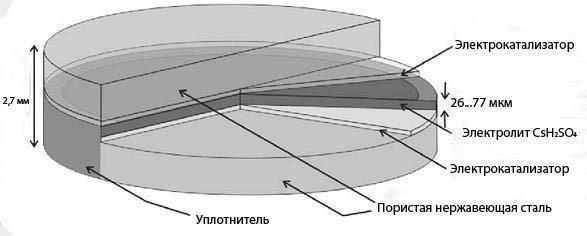
In tverdolystyh fuel cell electrolyte (CsHSO4) does not contain water. Therefore, the working temperature is 100-300°C. the Rotation of oxy anions SO42-allows the protons (red) to move as shown in the figure.
As a rule, tenacity fuel cell is a sandwich in which a very thin layer tertogennogo compound is located between two tightly clenched electrodes to ensure good contact. When heated, the organic component evaporates, leaving through the pores in the electrodes, while maintaining the ability of numerous contacts between the fuel (or by oxygen on the other end of the elements), electrolyte and electrodes.published
Type fuel cell operating temperature the Efficiency of electricity generation fuel Type Scope MCFC 550-700°C 50-70% of Most types of hydrocarbon fuel Medium and large installations, FCTE 100-220°C 35-40% Pure hydrogen Large installation MOPTA 30-100°C 35-50% Pure hydrogen Small fitting SOFC 450-1000°C 45-70% for Most types of hydrocarbon fuel Small, medium and large installation POMTE 20-90°C 20-30% Methanol Portable installation, STA 50-200°C 40-65% Pure hydrogen Space research, PETE 30-100°C 35-50%, Pure hydrogen is Small fitting
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: www.energy-units.ru/en_units.php
A fuel cell is similar to battery in that it produces direct current by chemical reaction. Again, like the battery, fuel elementvalue anode, cathode and electrolyte. However, unlike batteries, fuel cells can not accumulate electric energy, do not discharge and do not require electricity for re-charging. Fuel cells can continuously generate electricity as long as they have fuel and air. The correct term to describe the working of a fuel cell is a system of elements so as to complete the work required by the presence of some auxiliary systems.
Unlike other power generators such as internal combustion engines or turbines operating on gas, coal, oil etc., fuel cells do not burn fuel. This means no noisy high-pressure rotors, loud exhaust noise and vibrations. Fuel cells produce electricity through a silent electrochemical reaction. Another feature of fuel cells is that they convert the chemical energy of a fuel directly into electricity, heat and water.
Fuel cells are highly efficient and do not produce large amounts of greenhouse gases such as carbon dioxide, methane and nitrous oxide. The only product release from the fuel elements are water in the form of steam and small quantities of carbon dioxide, which is generally not allocated, if the fuel is pure hydrogen. The fuel elements are collected into assemblies, and then in a separate function modules.
The working principle of fuel cellsFuel cells produce electricity and heat due to the ongoing electrochemical reactions with the electrolyte, the cathode and anode.

The anode and cathode separated by electrolyte, conducting protons. After the hydrogen goes to the anode, and oxygen to the cathode, starts a chemical reaction, which generates electric current, heat and water. On the catalyst of the anode, molecular hydrogen dissociates and loses electrons. Hydrogen ions (protons) are conducted through the electrolyte to the cathode, while the electrons are ignored by the electrolyte and pass through the external circuit, creating a DC current that can be used to power the equipment. On the catalyst of the cathode, the oxygen molecule joins with an electron (which is supplied from an external communications) and came with a proton, and forms water, which is the only product of the reaction (in the form of steam and/or fluid).
The following is the response:
The reaction at the anode: 2H2 => 4H+ + 4e-
The reaction at the cathode: O2 + 4H+ + 4e- => 2H2O
The General reaction of the elements: 2H2 + O2 => 2H2O
Types of fuel cells

Like the existence of various types of internal combustion engines, there are different types of fuel cells – selection of suitable type of fuel cells depends on its application.Fuel cells are divided into high temperature and low temperature. Low-temperature fuel cells require the fuel is relatively pure hydrogen.
This often means that require fuel processing to convert the primary fuel (such as natural gas) into pure hydrogen. This process consumes extra energy and requires special equipment. High temperature fuel cells do not need this additional procedure, as they can implement "transformation" of the fuel at elevated temperatures, which means no need of investing money in hydrogen infrastructure.
Fuel cells molten carbonate (MCFC).

Fuel cells having molten carbonate electrolyte are high-temperature fuel cells. High operating temperature allows direct use of natural gas without the fuel processor and fuel gas with low calorific value fuel production processes and from other sources. This process was developed in the mid-1960s, Since that time has improved the technology of production, performance and reliability.
The work of the MCFC is different from other fuel cells. These elements use an electrolyte composed of a molten carbonate salt mixture. Currently, two types of compounds: lithium carbonate and potassium carbonate or lithium carbonate and sodium carbonate. For melting the carbonate salts and achieve high degree of mobility of ions in the electrolyte, the fuel cell with molten carbonate electrolyte occurs at high temperatures (650°C). Efficiency varies in the range of 60-80%.
When heated to a temperature of 650°C, the salt becomes a conductor of carbonate ions (CO32-). These ions pass from the cathode to the anode, where there is a combining with hydrogen to form water, carbon dioxide and free electrons. These electrons are directed through the external circuit back to the cathode, this will generate an electric current, and as a byproduct – heat.
The reaction on the anode: CO32- + H2 => H2O + CO2 + 2e-
The reaction at the cathode: CO2 + 1/2O2 + 2e- => CO32-
The General reaction of the elements: H2(g) + 1/2O2(g) + CO2(cathode) => H2O(g) + CO2(anode)
High operating temperature fuel cells with molten carbonate electrolyte have certain advantages. At high temperatures, internal reforming of natural gas, which eliminates the need for a fuel processor. In addition, the advantages include the possibility of using the standard materials of construction such as stainless steel sheets and Nickel catalyst on the electrodes. Product heat can be used to generate high pressure steam for various industrial and commercial purposes.
The high reaction temperature in the electrolyte also have their advantages. The use of high temperature requires significant time to achieve optimal operating conditions, the system responds more slowly to changes in energy consumption. These characteristics allow for installation on fuel cells with molten carbonate electrolyte under conditions of constant power. High temperatures prevent damage to the fuel cell carbon monoxide "poisoning", etc.
Fuel cells with molten carbonate electrolyte suitable for use in large stationary installations. Industrial produced heat and power installation with an output electrical power of 2.8 MW. Developed installation with output power up to 100 MW.
Fuel cells based on phosphoric acid (PCTA).

Fuel cells based on phosphoric (orthophosphoric) acid were the first fuel cells for commercial use. This process was developed in the mid-1960s, tests were conducted in the 1970s, Since that time has been increased stability, performance and reduced cost.
Fuel cells based on phosphoric (orthophosphoric) acid is used an electrolyte based on phosphoric acid (H3PO4) with a concentration of up to 100%. The ionic conductivity of phosphoric acid is low at low temperatures, for this reason, these fuel cells are used at temperatures of up to 150-220°C.
Carrier charge in the fuel cells of this type is the hydrogen (H+, proton). A similar process takes place in fuel cells with a membrane of exchange of protons (MOPTA), in which hydrogen supplied to the anode is split into protons and electrons. The protons pass through the electrolyte and combine with oxygen derived from air at the cathode to form water. The electrons are directed through the external circuit, this will generate an electric current. Below are reactions, which generate electric current and heat.
The reaction at the anode: 2H2 => 4H+ + 4e-
The reaction at the cathode: O2(g) + 4H+ + 4e- => 2H2O
The General reaction of the elements: 2H2 + O2 => 2H2O
Efficiency of fuel cells based on phosphoric (orthophosphoric) acid is more than 40% when generating electrical energy. The combined production of heat and electricity, total efficiency is about 85%. In addition, given the operating temperature, waste heat can be used for heating water and generating steam at atmospheric pressure.
High performance of thermal power plants on fuel cells based on phosphoric (orthophosphoric acid) for the combined production of heat and electric energy is one of the advantages of this type of fuel cells. The unit is a carbon monoxide concentration of about 1.5%, significantly expands the choice of fuel. In addition, CO2 does not affect the electrolyte and the fuel cell operation, the type of the elements works with reformirovaniyu natural fuels. Simple design, low degree of volatility of the electrolyte and high stability are the advantages of this type of fuel cells.
Industrial produced heat and power installation with an output electrical power up to 400 kW. Installation of 11 MW tested. Developed installation with output power up to 100 MW.
Fuel cells with a membrane of exchange of protons (MOPTA)

Fuel cells with a membrane of exchange of protons are considered the best type of fuel cells to generate power for vehicles capable of replacing petrol and diesel internal combustion engines. These fuel cells were first used by NASA to program "Gemini". Currently develop and demonstrate installation on MOPTA power from 1 watt to 2 kW.
The electrolyte in these fuel cells use solid polymer membrane (thin plastic film). When saturated with water this polymer transmits protons but does not conduct electrons.
The fuel is hydrogen, and the carrier charge – the hydrogen ion (proton). At the anode, the hydrogen molecule splits into a hydrogen ion (proton) and electrons. The hydrogen ions pass through the electrolyte to the cathode, while the electrons move around the outer circle and produce electrical energy. The oxygen is taken from air, is fed to the cathode and combines with the electrons and hydrogen ions to form water. At the electrodes the following reactions occur:
The reaction at the anode: 2H2 + 4OH- => 4H2O + 4e-
The reaction at the cathode: O2 + 2H2O + 4e- => 4OH-
The General reaction of the elements: 2H2 + O2 => 2H2O
Compared to other types of fuel cells, fuel cells with a membrane of exchange of protons produce more energy in a given volume or weight of fuel cell. This feature allows them to be compact and light. Besides, working temperature less than 100°C, which allows you to quickly start operation. These characteristics, as well as the ability to quickly change the output energy are just some of the features that make these fuel cells are the first candidate for use in vehicles.
Another advantage is that the electrolyte acts as a solid rather than a liquid substance. To keep the gases at both the anode and cathode is easier with the use of a solid electrolyte, and therefore, these fuel cells cheaper to produce. Compared to other electrolytes, the application of solid electrolyte does not occur such difficulties as the orientation, there is less of a problem due to corrosion, which leads to greater durability of the item and its components.
Solid oxide fuel cells (SOFC)

Solid oxide fuel cells are fuel cells with the highest operating temperature. Operating temperature can range from 600°C to 1000°C, which allows you to use different types of fuel without special pre-treatment. To work with such high temperatures used, the electrolyte is a thin solid metal oxide on a ceramic base, often an alloy of yttrium and zirconium, which is a conductor of oxygen ions (O2-). The technology of using solid oxide fuel cells developed since the late 1950s, and has two configurations: planar and tubular.
The solid electrolyte provides a sealed transfer of gas from one electrode to another, while liquid electrolytes are located in the porous substrate. Carrier charge in the fuel cells of this type is the oxygen ion (O2-). At the cathode, splitting the oxygen molecules from the air into oxygen ion and four electrons. The oxygen ions pass through the electrolyte and combine with hydrogen, this produces four free electrons. The electrons are directed through the external circuit, this will generate an electric current and waste heat.
The reaction at the anode: 2H2 + 2O2- => 2H2O + 4e-
The reaction at the cathode: O2 + 4e- => 2O2-
The General reaction of the elements: 2H2 + O2 => 2H2O
Efficiency of produced electric energy is the highest of all fuel cells is about 60%. In addition, the high operating temperatures allow for combined production of thermal and electric energy to generate high pressure steam. The combination of high temperature fuel cell with a turbine allows you to create hybrid fuel cell to increase the efficiency of generating electrical energy up to 70%.
Solid oxide fuel cells operate at very high temperatures (600°C–1000°C), which requires considerable time to achieve optimal operating conditions, the system responds more slowly to changes in energy consumption. At such high operating temperatures do not require the Converter to recover hydrogen from fuel thermal power plant to operate with a relatively impure fuels obtained through gasification of coal or exhaust, etc. Also, this fuel cell is particularly suited to high power, including industrial and large Central power plants. Industrial modules are available with output electric power of 100 kW.
Fuel cells with direct oxidation of methanol (PAMTA)
The technology of using fuel cells with direct oxidation of methanol is undergoing a period of active development. She has successfully established itself in the field of supply mobile phones, laptops, and also for creating portable power sources. which is the aim of future application of these elements.
Device fuel cell with direct oxidation of methanol are similar to fuel cells with a membrane of exchange of protons (MOPTA), i.e. in the electrolyte is a polymer and the charge carrier is the hydrogen ion (proton). However, liquid methanol (CH3OH) is oxidized in the presence of water at the anode to CO2, hydrogen ions and electrons that are directed through the external circuit, this will generate an electric current. The hydrogen ions pass through the electrolyte and react with oxygen from air and electrons supplied from external circuit to form water at the anode.
The reaction at the anode: CH3OH + H2O => CO2 + 6H+ + 6e-
The reaction at the cathode: 3/2O2 + 6H+ + 6e- => 3H2O
A common reaction of the element: CH3OH + 3/2O2 => CO2 + 2H2O
The development of these fuel cells was developed in the early 1990s, After the creation of improved catalysts and, thanks to another recent innovation has been increased power density and efficiency of up to 40%.
Tests were carried out in a temperature range of 50-120°C. Due to the low operating temperatures and no need to use Converter, fuel cells with direct oxidation of methanol is the best candidate for applications as in mobile phones and other consumer goods, and motor vehicles. The advantage of this type of fuel cells are small in size, due to the use of liquid fuel, and no need to use Converter.
Alkaline fuel cells (MTA)

Alkaline fuel cells (MTA) is one of the most proven technologies in use since the mid 1960s, the Agency NASA programs Apollo and the space Shuttle. Aboard these space ships, fuel cells produce electrical energy and drinking water. Alkaline fuel cells are one of the most effective elements used for the generation of electricity, the generation efficiency reaches 70%.
In alkaline fuel cells use an electrolyte that is an aqueous solution of potassium hydroxide contained in a porous stabilized matrix. The concentration of potassium hydroxide can vary depending on the operating temperature of the fuel cell, the range of which varies from 65°C to 220°C. the Carrier charge in STE is the hydroxyl ion (OH-), moving from the cathode to the anode, where it reacts with hydrogen to produce water and electrons. Water obtained on the anode, moves back to the cathode, there again generating hydroxyl ions. As a result of a number of reactions taking place in a fuel cell, produces electricity and, as byproduct, heat:
The reaction at the anode: 2H2 + 4OH- => 4H2O + 4e-
The reaction at the cathode: O2 + 2H2O + 4e- => 4OH-
Overall reaction: 2H2 + O2 => 2H2O
The advantage of STA is that these fuel cells are the cheapest to manufacture because the catalyst required on the electrodes can be any of the substances, cheaper than those used as catalysts for other fuel cells. In addition, STA work at relatively low temperatures and are among the most efficient fuel cells — such features may therefore contribute to the acceleration of generation of power and high fuel efficiency.
One of the characteristic features of STA – high sensitivity to CO2 that can be contained in the fuel or air. CO2 reacts with the electrolyte, poisons it rapidly, and severely reduces the efficiency of the fuel cell. Therefore, the use of STA is limited to closed spaces, such as space and underwater vehicles, they must operate on pure hydrogen and oxygen. Moreover, molecules like CO, H2O and CH4, which are safe for other fuel cells, and some of them even are fuels that are harmful to STA.
Polymer electrolyte fuel cells (PATE)

In the case of a polymer electrolyte fuel cell polymer membrane comprises polymer fibers with water areas where there is conductivity of water ions H2O+ (proton, red) attaches itself to the water molecule. Water molecules are a problem because of slow ion exchange. Therefore, it requires a high concentration of water in the fuel, and exhaust electrodes, which limits the working temperature of 100°C.
Tverdokopchenye fuel cells (TCTA)

In tverdolystyh fuel cell electrolyte (CsHSO4) does not contain water. Therefore, the working temperature is 100-300°C. the Rotation of oxy anions SO42-allows the protons (red) to move as shown in the figure.
As a rule, tenacity fuel cell is a sandwich in which a very thin layer tertogennogo compound is located between two tightly clenched electrodes to ensure good contact. When heated, the organic component evaporates, leaving through the pores in the electrodes, while maintaining the ability of numerous contacts between the fuel (or by oxygen on the other end of the elements), electrolyte and electrodes.published

Type fuel cell operating temperature the Efficiency of electricity generation fuel Type Scope MCFC 550-700°C 50-70% of Most types of hydrocarbon fuel Medium and large installations, FCTE 100-220°C 35-40% Pure hydrogen Large installation MOPTA 30-100°C 35-50% Pure hydrogen Small fitting SOFC 450-1000°C 45-70% for Most types of hydrocarbon fuel Small, medium and large installation POMTE 20-90°C 20-30% Methanol Portable installation, STA 50-200°C 40-65% Pure hydrogen Space research, PETE 30-100°C 35-50%, Pure hydrogen is Small fitting
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: www.energy-units.ru/en_units.php
Instead of yogurt for Breakfast...
Management of electronic socket: counter is no longer clocked extra electricity