513
Combating carbon: Can chemists turn dirt into gold?
We, the people, released of 35.9 metric gigatons of carbon dioxide into the atmosphere in 2014, mostly during combustion of coal and natural gas power plants, making fertilizer and cement, and other industrial Affairs. If chemists could capture carbon dioxide and turn it into chemical raw materials for other products, as do plants, "carbon dioxide would have become of the troubles as a gift," says chemical engineer at Cornell University Lynden Archer.
Sixty seven million eight hundred twelve thousand one hundred seventy
Scientists have been looking for a way to accumulate carbon dioxide captured from the chimneys of power plants and other facilities, lowering it deep beneath the earth. However, without significant subsidies, this expensive process of carbon capture may not be economically viable. It would be possible to launch carbon dioxide into old oil wells to force more oil, but not enough, and given the current low price of oil is still doubtful with a profit position. Supporters of using carbon instead of storing it hoped to profit by creating a useful product from useless. Most likely the use of gas as a raw material for the production of chemical products.
But such an option there are lots of chemical problems. Carbon dioxide is a stable molecule and does not store much energy in its chemical bonds. To use it, chemists have to add energy, usually by heating, which generally requires electricity. Electricity comes from power plants that burn coal or natural gas — as a result, the atmosphere will be released more uglekislogo than captured.
Engineers, chemists and other scientists say the new technology should change the rules of the game. Half-Potato, senior researcher of the group on energy and the environment at the XPrize Foundation, hopes that the promise of a large prize for the decision must be motivated to search a diverse group of technologists. Recently the Foundation announced that more than 40 team will compete for a prize of $ 20 million. The winner of the Carbon XPrize, which will be announced in the spring of 2020 will need to be able to recycle much of the carbon dioxide product with greater net worth. Some will try to make polymers, fuels to replace gasoline or industrial chemicals.
In the long run all companies that learn to do some sort of chemical substance, can come to a common decision that will change the recycling industry. The issue of large-scale climate change requires many solutions, says the Potato.
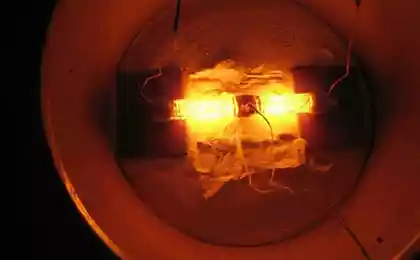
"The question now is how chemists will find new reactions and new mechanisms for use of carbon dioxide," goorit Archer, also a member of the Advisory Board of Carbon XPrize. This week in Science Advances, he suggested one option: the fuel cell that generates electric power by converting carbon dioxide into useful chemical. Archer and his student Vajji al Sadat had built a prototype reactor, which combines carbon dioxide with aluminum and produces oxalates. Oxalates are used for the production of acid, anti-rust, dyes for fabric and other industrial substances.
Thirty nine million two hundred forty eight thousand four hundred forty five
Archer is well aware of the pitfalls of trying to do organic chemistry with the use of carbon dioxide. "Usually you spend so much energy that the cost is too high — but we got our energy back. It surprised us".
The fuel cell works with aluminum and air. The oxygen reacts with the aluminum electrode to produce superoxide aluminum with high reactivity, capable of reacting with carbon dioxide. Their reaction leads to the appearance of oxalate of aluminium. Fuel cell gets energy from these chemical reactions, although the reaction requires electricity, but as it turned out, the process produces more energy than it produces. As it is metal, it is important to choose the appropriate one. Aluminium was chosen because it is a lot of it is inexpensive. Although the production of aluminium is released into the atmosphere dioxide ugleroda, Archer hopes his system will capture enough carbon to compensate for this.
Cornell group warns that she's not fully aware of involved chemical reactions. An early version of the fuel cell used is expensive — ionic liquid — the electrolyte. If it plays an important role and cannot be replaced, technology cannot be considered suitable, Archer says.
Oxalates — a niche product, like many of the chemicals produced by startups for the disposal of carbon. But some have more ambitious goals. Pilot plant Skyonic in San Antonio, Texas, captures the emissions from the cement plant and turn them into limestone and acid. Solidia Technologies sequestered carbon dioxide in the concrete. Other companies are working on the creation of plastics, alternative fuels and chemical raw materials.
Speaking without regard to any project, Howard Herzog believes that many of those that promise to capture carbon too sweet all describe. Herzog senior research engineer MIT Energy Initiative and a supporter of the carbon sequestration. "Carbon dioxide is wasted energy," he says. "From the point of view of energy impossible to win. This tells us thermodynamics," says Herzog.
Though Herzog recognizes that some of these companies can be profitable, he is sceptical about the possibility that recycling of carbon would have a significant impact on the environment. He was the lead author of the IPCC special report in 2005 said that the potential of recycling carbon is too weak to seriously change the picture of global emissions. Even if the chemical industry will do everything in their products from carbon dioxide — which is unlikely — it will not be able to absorb all emissions.
Kendra Kuhl, co-founder of Opus 12, the California startup, also believes that the disposal of carbon will completely solve the world's problems with the release. Opus 12 is developing an electrochemical reactor that uses new catalysts and renewable electricity to convert carbon dioxide into polymer building blocks and other chemicals. Kul says that the Opus 12 will fight for Carbon XPrize. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hi-news.ru/research-development/borba-s-uglerodom-mogut-li-ximiki-prevratit-gryaz-v-zoloto.html
Sixty seven million eight hundred twelve thousand one hundred seventy
Scientists have been looking for a way to accumulate carbon dioxide captured from the chimneys of power plants and other facilities, lowering it deep beneath the earth. However, without significant subsidies, this expensive process of carbon capture may not be economically viable. It would be possible to launch carbon dioxide into old oil wells to force more oil, but not enough, and given the current low price of oil is still doubtful with a profit position. Supporters of using carbon instead of storing it hoped to profit by creating a useful product from useless. Most likely the use of gas as a raw material for the production of chemical products.
But such an option there are lots of chemical problems. Carbon dioxide is a stable molecule and does not store much energy in its chemical bonds. To use it, chemists have to add energy, usually by heating, which generally requires electricity. Electricity comes from power plants that burn coal or natural gas — as a result, the atmosphere will be released more uglekislogo than captured.
Engineers, chemists and other scientists say the new technology should change the rules of the game. Half-Potato, senior researcher of the group on energy and the environment at the XPrize Foundation, hopes that the promise of a large prize for the decision must be motivated to search a diverse group of technologists. Recently the Foundation announced that more than 40 team will compete for a prize of $ 20 million. The winner of the Carbon XPrize, which will be announced in the spring of 2020 will need to be able to recycle much of the carbon dioxide product with greater net worth. Some will try to make polymers, fuels to replace gasoline or industrial chemicals.
In the long run all companies that learn to do some sort of chemical substance, can come to a common decision that will change the recycling industry. The issue of large-scale climate change requires many solutions, says the Potato.
"The question now is how chemists will find new reactions and new mechanisms for use of carbon dioxide," goorit Archer, also a member of the Advisory Board of Carbon XPrize. This week in Science Advances, he suggested one option: the fuel cell that generates electric power by converting carbon dioxide into useful chemical. Archer and his student Vajji al Sadat had built a prototype reactor, which combines carbon dioxide with aluminum and produces oxalates. Oxalates are used for the production of acid, anti-rust, dyes for fabric and other industrial substances.
Thirty nine million two hundred forty eight thousand four hundred forty five
Archer is well aware of the pitfalls of trying to do organic chemistry with the use of carbon dioxide. "Usually you spend so much energy that the cost is too high — but we got our energy back. It surprised us".
The fuel cell works with aluminum and air. The oxygen reacts with the aluminum electrode to produce superoxide aluminum with high reactivity, capable of reacting with carbon dioxide. Their reaction leads to the appearance of oxalate of aluminium. Fuel cell gets energy from these chemical reactions, although the reaction requires electricity, but as it turned out, the process produces more energy than it produces. As it is metal, it is important to choose the appropriate one. Aluminium was chosen because it is a lot of it is inexpensive. Although the production of aluminium is released into the atmosphere dioxide ugleroda, Archer hopes his system will capture enough carbon to compensate for this.
Cornell group warns that she's not fully aware of involved chemical reactions. An early version of the fuel cell used is expensive — ionic liquid — the electrolyte. If it plays an important role and cannot be replaced, technology cannot be considered suitable, Archer says.
Oxalates — a niche product, like many of the chemicals produced by startups for the disposal of carbon. But some have more ambitious goals. Pilot plant Skyonic in San Antonio, Texas, captures the emissions from the cement plant and turn them into limestone and acid. Solidia Technologies sequestered carbon dioxide in the concrete. Other companies are working on the creation of plastics, alternative fuels and chemical raw materials.
Speaking without regard to any project, Howard Herzog believes that many of those that promise to capture carbon too sweet all describe. Herzog senior research engineer MIT Energy Initiative and a supporter of the carbon sequestration. "Carbon dioxide is wasted energy," he says. "From the point of view of energy impossible to win. This tells us thermodynamics," says Herzog.
Though Herzog recognizes that some of these companies can be profitable, he is sceptical about the possibility that recycling of carbon would have a significant impact on the environment. He was the lead author of the IPCC special report in 2005 said that the potential of recycling carbon is too weak to seriously change the picture of global emissions. Even if the chemical industry will do everything in their products from carbon dioxide — which is unlikely — it will not be able to absorb all emissions.
Kendra Kuhl, co-founder of Opus 12, the California startup, also believes that the disposal of carbon will completely solve the world's problems with the release. Opus 12 is developing an electrochemical reactor that uses new catalysts and renewable electricity to convert carbon dioxide into polymer building blocks and other chemicals. Kul says that the Opus 12 will fight for Carbon XPrize. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hi-news.ru/research-development/borba-s-uglerodom-mogut-li-ximiki-prevratit-gryaz-v-zoloto.html