577
Chemists have found a way to turn CO2 into fuel
To get energy, usually need something to burn: conventional vehicles burn fuel in internal combustion engines, electric vehicles charge their batteries from electricity supplied, for example, in CHP plants burning natural gas, and even us for muscular or mental work necessary to "burn" inside eaten Breakfast.
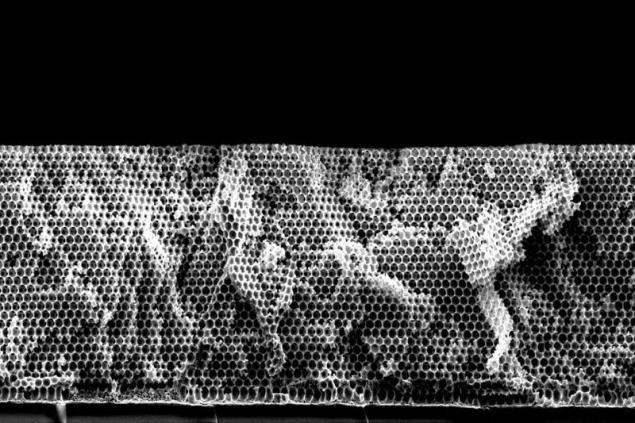
The porous structure of the surface of a silver catalyst after removal of the polystyrene matrix In any fossil fuel, be it petrol or hydrocarbons carbohydrates from chocolates, contain carbon atoms that are in the end energy pathways are transformed into carbon dioxide. Well, the gas, in turn, is sent into the atmosphere, where it can accumulate and cause all sorts of bad effects like global warming.
From the energy point of view, carbon dioxide is absolutely useless, because the carbon in it is completely "burned out" firmly and inextricably linking itself with two atoms of oxygen.
Burning he is not burning, and the only thing you can do with him – to drown or bury it. Drown it, dissolved in the ocean – and it really is one of the ways of disposing of CO2. Another way is to pump it under high pressure into the ground, preferably where there are oil fields; this will increase output from the oil reservoirs and will help to produce more oil.However, chemists have found a way to "cook porridge from an ax" – there is a third way of disposal of CO2 when it is converted into fuel.
To turn CO2 into fuel, "pohimichit" with a molecule of carbon dioxide, for example, to take away from it one atom of oxygen. Then the carbon dioxide turns into carbon monoxide, co. Despite the fact that for most carbon monoxide is "the gas, which periodically die sloppy users of wood-burning stoves", in industry it is used in different processes: first, it can burn and produce energy, and second, it can be used in metallurgical processes, and thirdly, it is possible to synthesize various organic molecules, including liquid fuels. Just the last paragraph and opens the carbon dioxide petrochemical prospects.
However, it should be noted that the use of carbon monoxide in the chemical order is not something entirely new. At the dawn of the twentieth century, German chemists Franz Fischer and Hans Tropsch developed a way of a conventional coal to liquid fuels: first coal and water into synthesis gas is a mixture of carbon monoxide and hydrogen, and then using the catalyst of the synthesis gas obtained various hydrocarbons.
This method was popular when regular oil is not enough, however, in the second half of the twentieth century, the method of producing fuel from coal has become just an expensive alternative to a "classic" oil refining technology. But if in the process of the Fischer-Tropsch process as raw materials use of coal, which in itself is minerals, the chemists from the Massachusetts Institute of technology for the same purpose – producing synthesis gas – has developed a way to make it "unnecessary" carbon dioxide.
Such things are not possible without the use of catalysts, and to obtain a working catalyst, chemists sometimes have to go to all sorts of tricks. The fact that, in addition to the specific chemical composition of the catalyst is very important to its internal structure. In simplistic terms, the catalyst deposited on a flat surface, may be non-functional, but if it is applied to a porous surface, and then this will be a certain size, then he'll earn in full force.
To create such a catalyst, the chemists took the conductive material as a substrate and had a layer of polystyrene balls with a diameter of about 200 nanometers. Then the voids remaining in the space between the balls filled with atoms of silver. (As an analogy one can imagine that we poured on the floor a layer of billiard balls, and then all poured on top of an even layer of melted paraffin.)
Now, to obtain a porous substrate, it is necessary somehow to remove from the material all the balls, leaving intact the remaining structure. In the case of pool balls it would be very difficult, but in the case of polystyrene balls it was a lot easier – and eventually after removal of the polystyrene on the surface of the electrode turned the cellular structure of silver with the "honeycombs" of a certain size.

Such material, as it turned out, well turns carbon dioxide into synthesis gas, and the efficiency and selectivity of the catalyst is controlled through the size of the cell: if at the stage of catalyst synthesis to take the polystyrene beads are larger, then the reaction will be one the composition of the products, and if smaller, then the other. Detailed results were published in the journal Angewandte Chemie.
And like all good, and humanity should celebrate the victory over greenhouse gas emissions, and each pipe, the Smoking in the atmosphere with products of combustion, it is necessary to equip such a silver catalyst, but it is worth to make a remark. One of the important laws on which there lives the world around us – the law of conservation: mass and energy can't appear from nowhere and do not disappear to nowhere. This is true for atoms of chemical elements, and the heat produced by the combustion of fuel and electrical energy.
So how much energy is obtained by burning carbon monoxide to carbon dioxide at least as much energy must be expended (simplified) to turn a molecule of carbon dioxide back to the carbon monoxide molecule. And obviously, for such, in General, "green" technology for recycling greenhouse gas needs a source of energy that is at least as "nachdi" in an atmosphere of much CO2 as could be turned into a useful product.
Where to get energy for the conversion of one gas to another? For example, from wind or solar power installations that produce energy but not emit products of combustion – as a result this would reduce the total amount of carbon dioxide.
It's funny that a similar activity was engaged ancient plants and bacteria, absorbs were then in abundance in the atmosphere of carbon dioxide, and preobrazovanie it into organic matter which later became a fossil fuel. It is possible that mankind in the future will have to do something similar, but only with the use of chemical technologies. published
Source: www.nkj.ru/news/30041/

The porous structure of the surface of a silver catalyst after removal of the polystyrene matrix In any fossil fuel, be it petrol or hydrocarbons carbohydrates from chocolates, contain carbon atoms that are in the end energy pathways are transformed into carbon dioxide. Well, the gas, in turn, is sent into the atmosphere, where it can accumulate and cause all sorts of bad effects like global warming.
From the energy point of view, carbon dioxide is absolutely useless, because the carbon in it is completely "burned out" firmly and inextricably linking itself with two atoms of oxygen.
Burning he is not burning, and the only thing you can do with him – to drown or bury it. Drown it, dissolved in the ocean – and it really is one of the ways of disposing of CO2. Another way is to pump it under high pressure into the ground, preferably where there are oil fields; this will increase output from the oil reservoirs and will help to produce more oil.However, chemists have found a way to "cook porridge from an ax" – there is a third way of disposal of CO2 when it is converted into fuel.
To turn CO2 into fuel, "pohimichit" with a molecule of carbon dioxide, for example, to take away from it one atom of oxygen. Then the carbon dioxide turns into carbon monoxide, co. Despite the fact that for most carbon monoxide is "the gas, which periodically die sloppy users of wood-burning stoves", in industry it is used in different processes: first, it can burn and produce energy, and second, it can be used in metallurgical processes, and thirdly, it is possible to synthesize various organic molecules, including liquid fuels. Just the last paragraph and opens the carbon dioxide petrochemical prospects.
However, it should be noted that the use of carbon monoxide in the chemical order is not something entirely new. At the dawn of the twentieth century, German chemists Franz Fischer and Hans Tropsch developed a way of a conventional coal to liquid fuels: first coal and water into synthesis gas is a mixture of carbon monoxide and hydrogen, and then using the catalyst of the synthesis gas obtained various hydrocarbons.
This method was popular when regular oil is not enough, however, in the second half of the twentieth century, the method of producing fuel from coal has become just an expensive alternative to a "classic" oil refining technology. But if in the process of the Fischer-Tropsch process as raw materials use of coal, which in itself is minerals, the chemists from the Massachusetts Institute of technology for the same purpose – producing synthesis gas – has developed a way to make it "unnecessary" carbon dioxide.
Such things are not possible without the use of catalysts, and to obtain a working catalyst, chemists sometimes have to go to all sorts of tricks. The fact that, in addition to the specific chemical composition of the catalyst is very important to its internal structure. In simplistic terms, the catalyst deposited on a flat surface, may be non-functional, but if it is applied to a porous surface, and then this will be a certain size, then he'll earn in full force.
To create such a catalyst, the chemists took the conductive material as a substrate and had a layer of polystyrene balls with a diameter of about 200 nanometers. Then the voids remaining in the space between the balls filled with atoms of silver. (As an analogy one can imagine that we poured on the floor a layer of billiard balls, and then all poured on top of an even layer of melted paraffin.)
Now, to obtain a porous substrate, it is necessary somehow to remove from the material all the balls, leaving intact the remaining structure. In the case of pool balls it would be very difficult, but in the case of polystyrene balls it was a lot easier – and eventually after removal of the polystyrene on the surface of the electrode turned the cellular structure of silver with the "honeycombs" of a certain size.

Such material, as it turned out, well turns carbon dioxide into synthesis gas, and the efficiency and selectivity of the catalyst is controlled through the size of the cell: if at the stage of catalyst synthesis to take the polystyrene beads are larger, then the reaction will be one the composition of the products, and if smaller, then the other. Detailed results were published in the journal Angewandte Chemie.
And like all good, and humanity should celebrate the victory over greenhouse gas emissions, and each pipe, the Smoking in the atmosphere with products of combustion, it is necessary to equip such a silver catalyst, but it is worth to make a remark. One of the important laws on which there lives the world around us – the law of conservation: mass and energy can't appear from nowhere and do not disappear to nowhere. This is true for atoms of chemical elements, and the heat produced by the combustion of fuel and electrical energy.
So how much energy is obtained by burning carbon monoxide to carbon dioxide at least as much energy must be expended (simplified) to turn a molecule of carbon dioxide back to the carbon monoxide molecule. And obviously, for such, in General, "green" technology for recycling greenhouse gas needs a source of energy that is at least as "nachdi" in an atmosphere of much CO2 as could be turned into a useful product.
Where to get energy for the conversion of one gas to another? For example, from wind or solar power installations that produce energy but not emit products of combustion – as a result this would reduce the total amount of carbon dioxide.
It's funny that a similar activity was engaged ancient plants and bacteria, absorbs were then in abundance in the atmosphere of carbon dioxide, and preobrazovanie it into organic matter which later became a fossil fuel. It is possible that mankind in the future will have to do something similar, but only with the use of chemical technologies. published
Source: www.nkj.ru/news/30041/
Egg shells can be used for the next generation of storage devices
As the autopilot goes through the city: live from the cockpit