487
Chinese chemists have learned to turn air into petrol
Chinese scientists have created a new catalyst based on iron nanoparticles, can "forever" to turn an ordinary carbon dioxide and hydrogen into a mixture of hydrocarbons similar to gasoline, according to a paper published in the journal Nature Communications.
"Over the past 200 years, coal, oil and gas were the main drivers of our civilization, the Foundation of its economic and social development. The burning of fuel resulted in the release of gigantic quantities of CO2 in the atmosphere that are causing negative climate change. The transformation of CO2 into fuels and chemicals will not only help us to cope with global warming, but also solve the problem of exhaustion of mineral resources", — say the sun Jian (Sun Jian) from the Institute of chemical physics in Dalian (China) and his colleagues.

In recent years, scientists are actively trying to find a way of turning atmospheric CO2 into biofuel and other useful substances. For example, in July last year, physicists from Chicago have designed a solar cell that uses light energy to split CO2 and production of carbon monoxide and hydrogen, and in October their colleagues from the National laboratory in Oak ridge have created a catalyst that converts the carbon dioxide in ordinary alcohol.
In principle, both can already be used for storing energy, however, these catalysts have two big drawbacks. They are easily damaged and require cleaning a few dozen hours of work, and also produce a lot of byproducts.
Sun and his team solved both these problems – the catalyst actually converts all of the carbon dioxide into hydrocarbons that form the basis of gasoline and other high octane fuels, and thus works at least 1,000 hours (six months) in "normal" industrial environments.
It consists of two components: nanoparticles from compounds of iron oxide and sodium, as well as so-called zeolites. Zeolites are hollow nanoparticles of aluminum silicate, which are now widely used for water purification and for the "packing" of the various catalysts, the penetration of the molecules inside the zeolites which significantly changes their properties and often causes them to behave much more active than in free form.
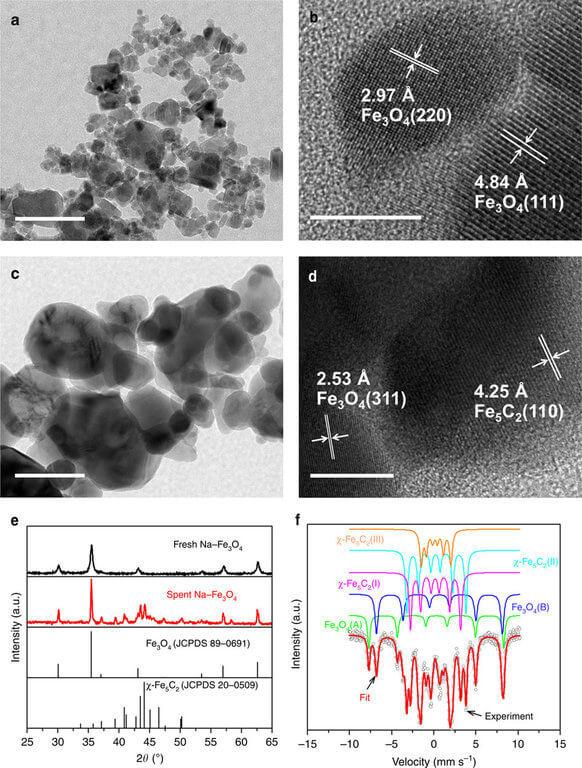
As scholars have noted, each component in this case plays a different role – iron nanoparticles "cut" the molecules of carbon dioxide and forced her to connect with atoms of hydrogen, and zeolites and their filling – contribute to the unification of these "foods" into long chains of hydrocarbons.
The combination of these components, according to Chinese chemists, allows us to achieve the actual "eternity" of such a catalyst. Its effectiveness, as scholars have noted, decreased by only 6% in the first 300 hours of work and then not changed, which suggests that it is stable and will remain that way for much longer than 1000 hours. In addition, 96% of the carbon dioxide is converted to the equivalent of gasoline, and only 4% CO2 is converted to methane.
Moreover, the "bouquet" of hydrocarbons can be flexibly changed by increasing or decreasing the proportion of hydrogen and CO2 in the mixture and varying the type of zeolite which is used as a "package" of iron nanoparticles. Using solar panels as energy source for heating the gas mixture and its flow through the catalyst, can effectively and cheap enough to store solar energy in the form familiar to all fuel without harm to the environment, sun sign and colleagues. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //www.energy-fresh.ru/news/?id=14326
"Over the past 200 years, coal, oil and gas were the main drivers of our civilization, the Foundation of its economic and social development. The burning of fuel resulted in the release of gigantic quantities of CO2 in the atmosphere that are causing negative climate change. The transformation of CO2 into fuels and chemicals will not only help us to cope with global warming, but also solve the problem of exhaustion of mineral resources", — say the sun Jian (Sun Jian) from the Institute of chemical physics in Dalian (China) and his colleagues.

In recent years, scientists are actively trying to find a way of turning atmospheric CO2 into biofuel and other useful substances. For example, in July last year, physicists from Chicago have designed a solar cell that uses light energy to split CO2 and production of carbon monoxide and hydrogen, and in October their colleagues from the National laboratory in Oak ridge have created a catalyst that converts the carbon dioxide in ordinary alcohol.
In principle, both can already be used for storing energy, however, these catalysts have two big drawbacks. They are easily damaged and require cleaning a few dozen hours of work, and also produce a lot of byproducts.
Sun and his team solved both these problems – the catalyst actually converts all of the carbon dioxide into hydrocarbons that form the basis of gasoline and other high octane fuels, and thus works at least 1,000 hours (six months) in "normal" industrial environments.
It consists of two components: nanoparticles from compounds of iron oxide and sodium, as well as so-called zeolites. Zeolites are hollow nanoparticles of aluminum silicate, which are now widely used for water purification and for the "packing" of the various catalysts, the penetration of the molecules inside the zeolites which significantly changes their properties and often causes them to behave much more active than in free form.

As scholars have noted, each component in this case plays a different role – iron nanoparticles "cut" the molecules of carbon dioxide and forced her to connect with atoms of hydrogen, and zeolites and their filling – contribute to the unification of these "foods" into long chains of hydrocarbons.
The combination of these components, according to Chinese chemists, allows us to achieve the actual "eternity" of such a catalyst. Its effectiveness, as scholars have noted, decreased by only 6% in the first 300 hours of work and then not changed, which suggests that it is stable and will remain that way for much longer than 1000 hours. In addition, 96% of the carbon dioxide is converted to the equivalent of gasoline, and only 4% CO2 is converted to methane.
Moreover, the "bouquet" of hydrocarbons can be flexibly changed by increasing or decreasing the proportion of hydrogen and CO2 in the mixture and varying the type of zeolite which is used as a "package" of iron nanoparticles. Using solar panels as energy source for heating the gas mixture and its flow through the catalyst, can effectively and cheap enough to store solar energy in the form familiar to all fuel without harm to the environment, sun sign and colleagues. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: //www.energy-fresh.ru/news/?id=14326