975
Lithium-ion batteries is 25 years old
This year marks 25 years since the release of the first lithium-ion batteries, which produced the Sony Corporation in 1991. A quarter of a century, their capacity has nearly doubled from 110 WH/kg to 200 WH/kg, but despite such tremendous progress and numerous studies of electrochemical mechanisms, chemical processes and materials within lithium-ion batteries are virtually the same as 25 years ago. In this article you will learn how was the formation and development of this technology and the challenges facing developers of new materials.

1. The development of the technology: 1980-2000
Back in the 70s, scientists have established that there are materials called chalcogenides (e.g., MoS2), which can enter into reversible reaction with lithium ions by integrating them into its layered crystal structure. Was immediately offered the first prototype of the lithium-ion battery consisting of chalcogenides on the cathode and lithium metal on the anode. Theoretically, during discharge, lithium ions, "released" anode shall be embedded in the layered structure of MoS2, and when charging to settle back at the anode, returning to its original state.
But the first attempts of creation of such batteries were unsuccessful as when charging, lithium ions did not want to turn back into a flat plate of metallic lithium, and settled on the anode as a hit, leading to growth of dendrites (chains of lithium metal), short circuit and explosion of battery. This was followed by a stage of detailed study of the reaction of intercalating (embedding of lithium in crystals with special structure) that allowed for the replacement of metallic lithium on the carbon: first coke, and graphite, which is used until now and also has a layered structure that is able to incorporate lithium ions.
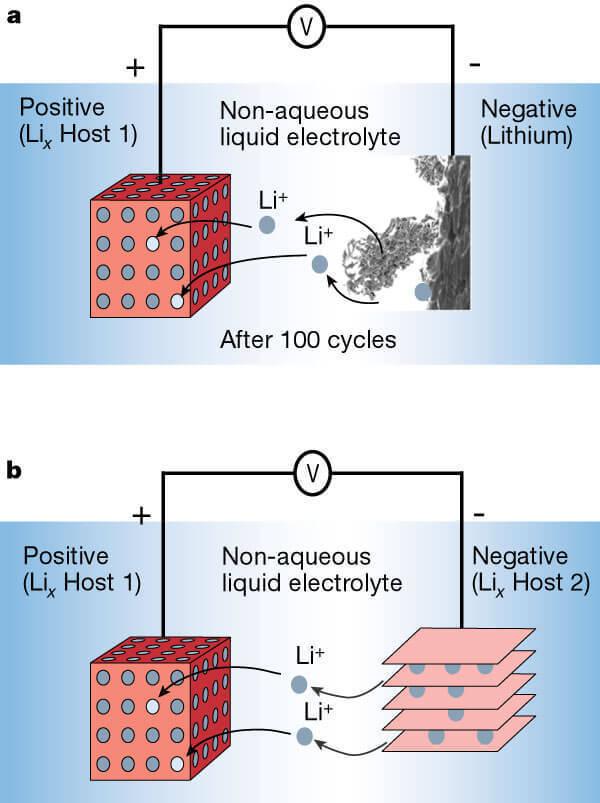
Li-ion battery with anode of lithium metal (a) and the anode of the layered material (b).
Starting to use carbon materials for the anode, the scientists realized that nature has done humanity a great gift. For graphite, when the very first charge, forms a protective layer of decomposed electrolyte, called SEI (Solid Electrolyte Interface). The exact mechanism of its formation and composition is still not fully understood, but we know that without this unique passivation layer, the electrolyte continues to decompose at the anode electrode would be destroyed, and the battery would come into disrepair. So there was the first working anode based on carbon materials, which was released in the composition of the lithium-ion batteries in the 90-ies.
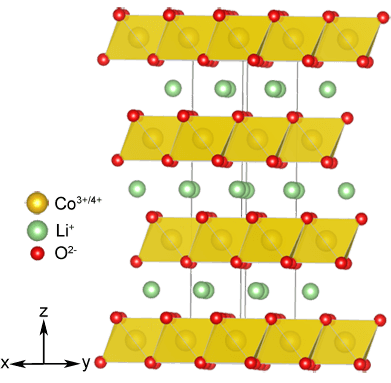
Simultaneously with the modified anode and cathode: it turned out that a layered structure is able to incorporate lithium ions, have not only chalcogenides, and certain transition metal oxides, such as LiMO2 ( M = Ni, Co, Mn), which is not only more stable chemically, but also allow to create cells with higher voltage. And that LiCoO2 was used in the cathode of the first commercial prototype batteries.
2. New reactions and the fashion for nanomaterials: 2000-2010
In the 2000s the science boom of nanomaterials. Of course, progress in nanotechnology is not spared and lithium-ion batteries. And thanks to them, scientists have made absolutely would seem unsuitable for this technology material, LiFePO4, one of the leaders in the use of cathodes electric batteries.
But the thing is that the ordinary, three-dimensional particles Jelisaveta very bad conduct ions, and electronic conductivity are very low. But due to a litany of nanostructuring is not necessary to move large distances to fit into the nanocrystal, so intercalation is a lot faster and the coating of nanocrystals with a thin carbon film improves its conductivity. Resulted in selling out is not only less hazardous material that does not emit oxygen at a high temperature (as oxides), but also a material having the ability to operate at higher currents. Therefore, such a cathode material manufacturers prefer a car, despite a slightly lower capacity than LiCoO2.
At the same time, scientists searched for new materials that interact with lithium. And, as it turned out, the intercalation or embedding of the lithium chip is not the only option reactions at the electrodes in lithium-ion batteries. For example, some elements, namely Si, Sn, Sb, etc., form an "alloy" with lithium, when used in the anode. The capacity of this electrode is 10 times the capacity of graphite, but there is one "but": such an electrode during the formation of the alloy greatly increases in volume, which leads to rapid cracking and coming into disrepair. And in order to reduce mechanical stress of the electrode with this increase, the element (e.g., silicon) propose to use in the form of nanoparticles enclosed in carbon matrix that "absorbs" the changes in the volume.
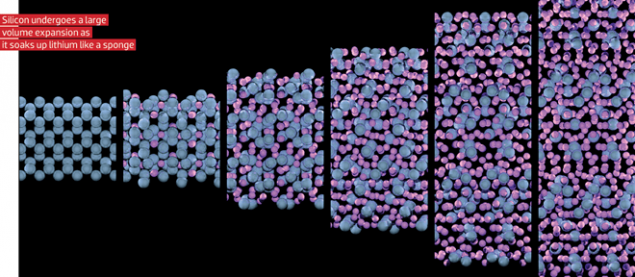
But change volume is not the only problem of the materials forming the alloys, and to prevent their widespread application. As mentioned above, graphite is formed "gift of nature"- SEI. And the materials forming the alloy, the electrolyte is decomposed continuously and increases the resistance of the electrode. But nevertheless, periodically we see in the news that some batteries use "a silicon anode". Yes, the silicon in it is used, but in very small quantities and mixed with graphite to side effects were not too noticeable. Naturally, when the amount of silicon in the anode is only a few percent, and the rest - graphite, a significant increase in capacity will not work.
And if the subject of the anodes that form alloys, is now developing, some research started in the last decade, very quickly came to a standstill. This applies, for example, the so-called conversion reactions. In this reaction, some compounds of metals (oxides, nitrides, sulfides, etc.) interact with lithium, turning into metal, mixed with lithium compounds:
MaXb ==> aM + bLinX
M: metal
X: O, N, C, S...
And, as you can imagine, with the material during such reactions occur such changes, which even the silicon not dreamed of. For example, cobalt oxide turns into cobalt metal nanoparticles that are enclosed in a matrix of oxides of lithium:
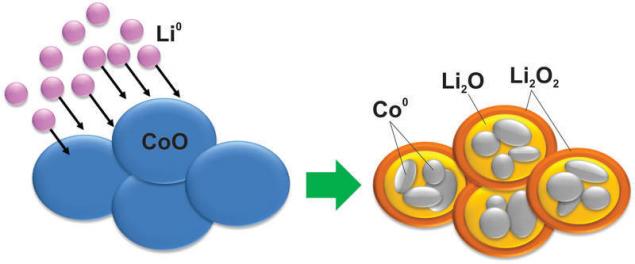
Naturally, this reaction poorly reversible to the same between charging and discharging a large voltage difference of, making these materials useless in application.
It is interesting to note that when this reaction was opened in the scientific journals began to publish hundreds of articles on this topic. But then hochetsja to quote Professor Tarascon from the Collège de France, who said that "the reaction conversion was a true field experiments for studies of materials with nanoarchitectures that gave scientists the ability to make beautiful images using transmission electron microscope and published in renowned journals, despite the absolute practical uselessness of these materials."
In General, to sum up, despite the fact that in the last decade were synthesized hundreds of new materials for electrodes in batteries even used almost the same materials as 25 years ago. Why did it happen?
3. Present: challenges in the development of new batteries.
As you can see in the above excursus into the history of lithium-ion batteries, not a word was said about another, the most important element: the electrolyte. And there's a reason: the electrolyte for 25 years practically has not changed and working alternatives was proposed. Today, as in 90-e years, in the form of an electrolyte used, lithium salt (mainly LiPF6) in an organic solution of the carbonates (ethylene carbonate (EC) + dimethyl carbonate (DMC)). But because of the electrolyte progress in increasing battery capacity in recent years has slowed.
Here is an example: today there are materials for electrodes, which could greatly increase the capacity of lithium-ion batteries. These include, for example, LiNi0.5Mn1.5О4, which would make the battery voltage of the cell at 5 Volts. But alas, in the voltage range of the electrolyte based on carbonate becomes unstable. Or another example: as mentioned above, today to use a significant amount of silicon (or other metals forming alloys with lithium) in the anode, it is necessary to solve one of the main problems: the formation of a passivation layer (SEI) which would prevent the continuous decomposition of electrolyte and destruction of the electrode, and for this we need to develop a fundamentally new composition of the electrolyte. But why is it so difficult to find an alternative to the existing structure, after all, full of lithium salts, and organic solvents enough?!
And the challenge constitutes the fact that the electrolyte should simultaneously possess the following characteristics:
In parallel with the improvement of existing technologies, scientists are working on a fundamentally new solutions. And these decisions can be reduced to attempt to get rid of liquid solvent on the basis of carbonates. These technologies include, for example, ionic liquids. Ionic liquids is essentially molten salts with very low melting point, and some of them even at room temperature remains liquid. And all because of the fact that these special salts, sterically hindered structure, which will complicate the crystallization.
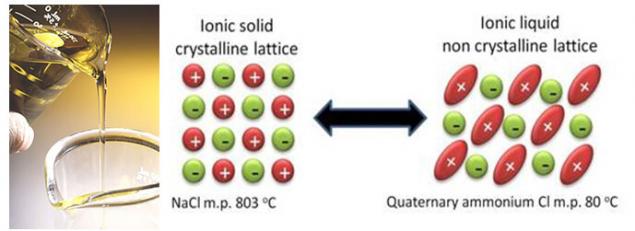
It seemed like a great idea to completely eliminate the solvent, which is flammable, and reacts to parasitic reactions with the lithium. But in fact, elimination of solvent creates at the moment more problems than it solves. First, in the usual electrolytes of the solvent "sacrifices himself" to build a protective layer on the electrode surface. As components of ionic liquids with this task I haven't yet (anions, incidentally, can also engage in parasitic reactions with the electrodes, as solvents). Secondly, it is very difficult to select the ionic liquid with the correct anion, as they affect not only the melting point of the salt but also on the electrochemical stability. And alas, the most stable anions to form salts that melt at high temperatures, and Vice versa.
Another way to get rid of the solvent on the basis of carbonates - the use of solid polymers (e.g., polyesters) conducting lithium, which, firstly, minimise the risk of leakage of the electrolyte out, and would prevent the growth of dendrites when using a metallic lithium on the anode. But the main problem facing the creators of polymer electrolytes is their very low ionic conductivity, since lithium ions are difficult to move in such a viscous medium. This, of course, greatly limits the power of the batteries. And lower viscosity entails the sprouting of dendrites.
The researchers also study of solid inorganic substances that conduct lithium through defects in the crystal, and try to apply them in the form of electrolytes for lithium-ion batteries. This system at first glance is perfect: chemical and electrochemical stability, resistance to temperature rise and mechanical strength. But these materials, again, very low ionic conductivity, and use them celesoobrazno only in the form of thin films. Besides, such materials are best at high temperature. And last, with a solid electrolyte is very difficult to create mechanical contact between Electrica and the electrodes (in the field of liquid electrolytes has no equal).
4. Conclusion.
Since the introduction of lithium-ion batteries, trying to increase their capacity does not stop. But in recent years the increase in capacity has slowed, despite the hundreds of proposed new materials for the electrodes. But the thing is that most of these new materials "sitting on shelf" waiting until a new, suitable electrolyte. And the development of new electrolytes - in my opinion much more complex task than the development of new electrodes, as the need to consider not only the electrochemical properties of the electrolyte, but also for its interaction with the electrodes. In General, reading news such as "developed a new super-electrode..." should be checked, such as the electrode communicates with the electrolyte, and is there for this electrode the electrolyte is suitable in principle. published
Source: geektimes.ru/post/282424/

1. The development of the technology: 1980-2000
Back in the 70s, scientists have established that there are materials called chalcogenides (e.g., MoS2), which can enter into reversible reaction with lithium ions by integrating them into its layered crystal structure. Was immediately offered the first prototype of the lithium-ion battery consisting of chalcogenides on the cathode and lithium metal on the anode. Theoretically, during discharge, lithium ions, "released" anode shall be embedded in the layered structure of MoS2, and when charging to settle back at the anode, returning to its original state.
But the first attempts of creation of such batteries were unsuccessful as when charging, lithium ions did not want to turn back into a flat plate of metallic lithium, and settled on the anode as a hit, leading to growth of dendrites (chains of lithium metal), short circuit and explosion of battery. This was followed by a stage of detailed study of the reaction of intercalating (embedding of lithium in crystals with special structure) that allowed for the replacement of metallic lithium on the carbon: first coke, and graphite, which is used until now and also has a layered structure that is able to incorporate lithium ions.

Li-ion battery with anode of lithium metal (a) and the anode of the layered material (b).
Starting to use carbon materials for the anode, the scientists realized that nature has done humanity a great gift. For graphite, when the very first charge, forms a protective layer of decomposed electrolyte, called SEI (Solid Electrolyte Interface). The exact mechanism of its formation and composition is still not fully understood, but we know that without this unique passivation layer, the electrolyte continues to decompose at the anode electrode would be destroyed, and the battery would come into disrepair. So there was the first working anode based on carbon materials, which was released in the composition of the lithium-ion batteries in the 90-ies.
Simultaneously with the modified anode and cathode: it turned out that a layered structure is able to incorporate lithium ions, have not only chalcogenides, and certain transition metal oxides, such as LiMO2 ( M = Ni, Co, Mn), which is not only more stable chemically, but also allow to create cells with higher voltage. And that LiCoO2 was used in the cathode of the first commercial prototype batteries.

2. New reactions and the fashion for nanomaterials: 2000-2010
In the 2000s the science boom of nanomaterials. Of course, progress in nanotechnology is not spared and lithium-ion batteries. And thanks to them, scientists have made absolutely would seem unsuitable for this technology material, LiFePO4, one of the leaders in the use of cathodes electric batteries.
But the thing is that the ordinary, three-dimensional particles Jelisaveta very bad conduct ions, and electronic conductivity are very low. But due to a litany of nanostructuring is not necessary to move large distances to fit into the nanocrystal, so intercalation is a lot faster and the coating of nanocrystals with a thin carbon film improves its conductivity. Resulted in selling out is not only less hazardous material that does not emit oxygen at a high temperature (as oxides), but also a material having the ability to operate at higher currents. Therefore, such a cathode material manufacturers prefer a car, despite a slightly lower capacity than LiCoO2.
At the same time, scientists searched for new materials that interact with lithium. And, as it turned out, the intercalation or embedding of the lithium chip is not the only option reactions at the electrodes in lithium-ion batteries. For example, some elements, namely Si, Sn, Sb, etc., form an "alloy" with lithium, when used in the anode. The capacity of this electrode is 10 times the capacity of graphite, but there is one "but": such an electrode during the formation of the alloy greatly increases in volume, which leads to rapid cracking and coming into disrepair. And in order to reduce mechanical stress of the electrode with this increase, the element (e.g., silicon) propose to use in the form of nanoparticles enclosed in carbon matrix that "absorbs" the changes in the volume.

But change volume is not the only problem of the materials forming the alloys, and to prevent their widespread application. As mentioned above, graphite is formed "gift of nature"- SEI. And the materials forming the alloy, the electrolyte is decomposed continuously and increases the resistance of the electrode. But nevertheless, periodically we see in the news that some batteries use "a silicon anode". Yes, the silicon in it is used, but in very small quantities and mixed with graphite to side effects were not too noticeable. Naturally, when the amount of silicon in the anode is only a few percent, and the rest - graphite, a significant increase in capacity will not work.
And if the subject of the anodes that form alloys, is now developing, some research started in the last decade, very quickly came to a standstill. This applies, for example, the so-called conversion reactions. In this reaction, some compounds of metals (oxides, nitrides, sulfides, etc.) interact with lithium, turning into metal, mixed with lithium compounds:
MaXb ==> aM + bLinX
M: metal
X: O, N, C, S...
And, as you can imagine, with the material during such reactions occur such changes, which even the silicon not dreamed of. For example, cobalt oxide turns into cobalt metal nanoparticles that are enclosed in a matrix of oxides of lithium:

Naturally, this reaction poorly reversible to the same between charging and discharging a large voltage difference of, making these materials useless in application.
It is interesting to note that when this reaction was opened in the scientific journals began to publish hundreds of articles on this topic. But then hochetsja to quote Professor Tarascon from the Collège de France, who said that "the reaction conversion was a true field experiments for studies of materials with nanoarchitectures that gave scientists the ability to make beautiful images using transmission electron microscope and published in renowned journals, despite the absolute practical uselessness of these materials."
In General, to sum up, despite the fact that in the last decade were synthesized hundreds of new materials for electrodes in batteries even used almost the same materials as 25 years ago. Why did it happen?
3. Present: challenges in the development of new batteries.
As you can see in the above excursus into the history of lithium-ion batteries, not a word was said about another, the most important element: the electrolyte. And there's a reason: the electrolyte for 25 years practically has not changed and working alternatives was proposed. Today, as in 90-e years, in the form of an electrolyte used, lithium salt (mainly LiPF6) in an organic solution of the carbonates (ethylene carbonate (EC) + dimethyl carbonate (DMC)). But because of the electrolyte progress in increasing battery capacity in recent years has slowed.
Here is an example: today there are materials for electrodes, which could greatly increase the capacity of lithium-ion batteries. These include, for example, LiNi0.5Mn1.5О4, which would make the battery voltage of the cell at 5 Volts. But alas, in the voltage range of the electrolyte based on carbonate becomes unstable. Or another example: as mentioned above, today to use a significant amount of silicon (or other metals forming alloys with lithium) in the anode, it is necessary to solve one of the main problems: the formation of a passivation layer (SEI) which would prevent the continuous decomposition of electrolyte and destruction of the electrode, and for this we need to develop a fundamentally new composition of the electrolyte. But why is it so difficult to find an alternative to the existing structure, after all, full of lithium salts, and organic solvents enough?!
And the challenge constitutes the fact that the electrolyte should simultaneously possess the following characteristics:
- It needs to be chemically stable in the battery life, or rather, it needs to be resistant to oxidizing the cathode and restoring the anode. This means that attempts to increase the power consumption of the battery, that is, the use of a more oxidizing cathode and reducing the anode, should not lead to decomposition of the electrolyte.
- The electrolyte must have good ionic conductivity and low viscosity to transport the lithium ions in a wide range of temperatures. To this viscous ethylene carbonate is added DMC since 1994.
- Lithium salts should be well dissolved in an organic solvent.
- The electrolyte should form an effective passivating layer. The ethylene carbonate is well obtained, while other solvents such as propylene carbonate, which was initially tested Sony destroys the structure of the anode, as it is embedded in parallel with the lithium.
In parallel with the improvement of existing technologies, scientists are working on a fundamentally new solutions. And these decisions can be reduced to attempt to get rid of liquid solvent on the basis of carbonates. These technologies include, for example, ionic liquids. Ionic liquids is essentially molten salts with very low melting point, and some of them even at room temperature remains liquid. And all because of the fact that these special salts, sterically hindered structure, which will complicate the crystallization.

It seemed like a great idea to completely eliminate the solvent, which is flammable, and reacts to parasitic reactions with the lithium. But in fact, elimination of solvent creates at the moment more problems than it solves. First, in the usual electrolytes of the solvent "sacrifices himself" to build a protective layer on the electrode surface. As components of ionic liquids with this task I haven't yet (anions, incidentally, can also engage in parasitic reactions with the electrodes, as solvents). Secondly, it is very difficult to select the ionic liquid with the correct anion, as they affect not only the melting point of the salt but also on the electrochemical stability. And alas, the most stable anions to form salts that melt at high temperatures, and Vice versa.
Another way to get rid of the solvent on the basis of carbonates - the use of solid polymers (e.g., polyesters) conducting lithium, which, firstly, minimise the risk of leakage of the electrolyte out, and would prevent the growth of dendrites when using a metallic lithium on the anode. But the main problem facing the creators of polymer electrolytes is their very low ionic conductivity, since lithium ions are difficult to move in such a viscous medium. This, of course, greatly limits the power of the batteries. And lower viscosity entails the sprouting of dendrites.

The researchers also study of solid inorganic substances that conduct lithium through defects in the crystal, and try to apply them in the form of electrolytes for lithium-ion batteries. This system at first glance is perfect: chemical and electrochemical stability, resistance to temperature rise and mechanical strength. But these materials, again, very low ionic conductivity, and use them celesoobrazno only in the form of thin films. Besides, such materials are best at high temperature. And last, with a solid electrolyte is very difficult to create mechanical contact between Electrica and the electrodes (in the field of liquid electrolytes has no equal).
4. Conclusion.
Since the introduction of lithium-ion batteries, trying to increase their capacity does not stop. But in recent years the increase in capacity has slowed, despite the hundreds of proposed new materials for the electrodes. But the thing is that most of these new materials "sitting on shelf" waiting until a new, suitable electrolyte. And the development of new electrolytes - in my opinion much more complex task than the development of new electrodes, as the need to consider not only the electrochemical properties of the electrolyte, but also for its interaction with the electrodes. In General, reading news such as "developed a new super-electrode..." should be checked, such as the electrode communicates with the electrolyte, and is there for this electrode the electrolyte is suitable in principle. published
Source: geektimes.ru/post/282424/
Diamond batteries will transform the radioactive waste into clean energy
SolarStratos: a solar-powered aircraft, which will rise into the stratosphere