860
An invisible axis: How our gut talks to our brain
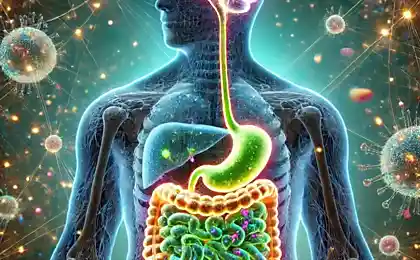
Smell the insides? Then you fluttering butterflies in a stomach, one that sucks in his stomach from fear, the bear develops a disease with severe anxiety. Familiar? Today we will talk about the connection between brain and intestines. Yes, the gut has many nerve cells, many bacteria that affect our brain much stronger than we think. The average person has approximately 1.5 kilograms of gut bacteria.
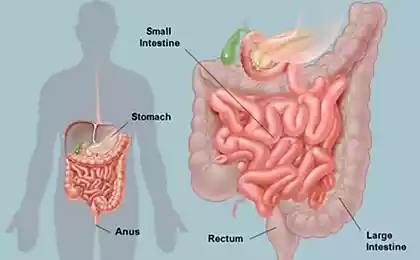
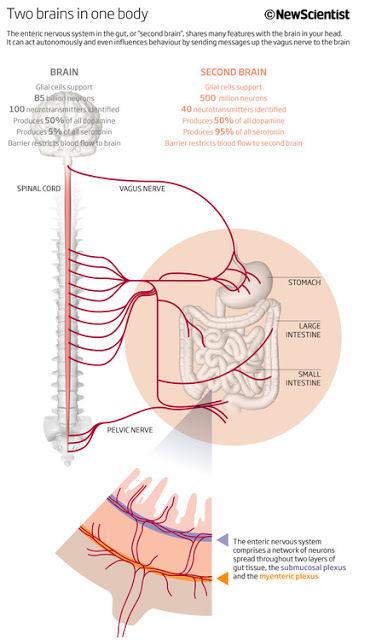
A so-called enteric nervous system, located between the esophagus and the intestine, consists of 100 million nerve cells. Please note: there are more of them than in the spinal cord. This is the second complex cluster of nerves in the human body after the brain. Our brain, with all its feelings, emotions and thoughts constantly communicates with "kisacanin brain." This process of communication is called "the axis brain – gut".
Remember that healthy nutrition is half of health. And healthy diet includes the impact of foods on our little intestinal friends. Remember that food is not just calories and energy. Food contains information, which she tells to your genes, turning on and off them, moment to moment, influencing their function. Food is the most powerful and fast-acting medicine that you can take to change your life. Food is not only calories. This is the information. It tells the genes what to do (and not do).
What is the axis of the gut-brain?
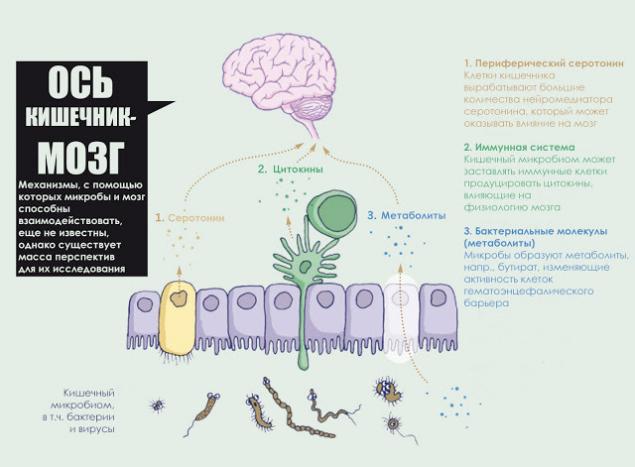
The axis the “gut-brain” – an imaginary line connected and one of the new horizons complex neuroscience. Gut microbiota (or microflora), which is often now called the “second genome” and the “second brain,” may influence our mood through mechanisms that scientists are only beginning to understand. And, unlike the genes that we inherit, the microflora can be changed and even grown. As soon as studies are transferred from mice not people, we are getting more understanding of the relationships of microflora our brains become visible an important connection with the mental (or emotional) health. A Japanese magnate was once asked how he will know whether to join the deal and he replied: "I swallow it, and if I like the feeling in my stomach, I enter into a transaction". Our gut with your own mind, but is continuously talking to our brain.
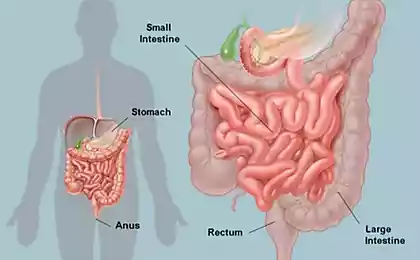
Digestion is a complex process, so there is nothing surprising in the fact that for its regulation there is a separate neural network. Digestive the nervous system is responsible for the processes of mechanical mixing of food in the stomach, coordinating the contraction of circular muscles and all of the sphincter throughout the bowel to ensure the progress of food, it also supports different biochemical environment and the level of acidity inside each individual section of the digestive tract, enabling the enzymes necessary conditions for their work.
Do not have to be a gastroenterologist to be aware of these reactions, or may be a more subtle feeling in my stomach that accompany emotions such as anxiety, excitement, fear or stress. For millennia, people were convinced that the gastrointestinal tract is associated with the brain and affect health. Only in the last century this relationship has been extensively studied. Two pioneers in this field was the American physician B. Robinson (published in 1907 his work titled "the Abdominal and Pelvic Brain") and his contemporary British physiologist Ivan Langley, who coined the term "gastro-intestinal nervous system".
In the early twentieth century Englishman Newport Langley calculated the number of nerve cells in the stomach and intestines — 100 million. More than in the spinal cord! There are no hemispheres, but in the presence of a branched network of neurons and supporting cells along with all sorts of impulses and signals. It has been suggested: is it possible to count such a conglomeration of nerve cells a kind of "ventral" brain?
The intestinal brain.
Recently on this subject said the Professor of neurogastroenterology Paul Enk from the University of Tubingen: "the Brain the stomach is about the same as the head. It can be represented in the form of a stocking which covers the esophagus, stomach and intestines. In the stomach and intestines of people suffering from Alzheimer's and Parkinson's, discovered the same tissue damage as in the brain. Therefore, the antidepressants like prozac that affect on the stomach."
A decade after the release of the most popular works of the "Second brain" American scientist confirms the assumption that the nervous system of the intestine is not stupid cluster nodes and tissues that executes commands of the Central nervous system, according to an old medical doctrine, and a unique network able to carry out complex processes on their own.
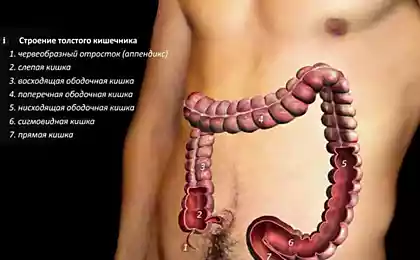
It is noteworthy that the gut continues to function even when there is no communication with the brain and spinal cord. Intestinal brain to decide all aspects of digestion throughout the gastrointestinal tract — from the esophagus to the intestine and rectum. They used the same tools as the "noble" brain: a whole web of neural circuits, neurotransmitters and proteins. Evolution is evidence of his insight: instead of trying to force the head of the severely strain the activity of millions of nerve cells to communicate with a remote site of the body, she chose to entrust the management of the center, located in controlled areas.
According to modern concepts, the neurotransmitters produced by the neurons of the gastrointestinal tract, are not able to get into the brain, however they are still unable to penetrate a small area of the brain where the blood-brain barrier permeability is higher, for example, in the hypothalamus. Whatever it was, nervous signals sent from the gastrointestinal tract to the brain, no doubt, affect the mood. Researchers began to decipher the ways in which gut bacteria can signal the brain. Peterson and others have shown that in adult mice microbial metabolites affect the basic physiology of the blood-brain barrier. Gut microbes break down complex carbohydrates to short chain fatty acids with the formation of a mass of effects: the fatty acid butyrate, for example, strengthen the blood-brain barrier, “tightening” the connections between cells.
Coexistence of symbiotic microflora and its media, for the most part, mutually beneficial. In particular, the presence of symbionts is essential for the functioning of our immune system, digest nutrients and other aspects of healthy physiology. Using the most modern tools to study genetics and tissues at the molecular level, scientists were able to demonstrate that in the gut are a few types of bacteria, and that symbiotic populations are characterized by great variety: you can allocate up to thousands of different species. In addition, the formation of individual microflora is constantly influenced by such factors as gender, genetics, age, type of food .
In healthy people, biological diversity is much more but at the same time, studying the microflora of these people in different moments of time (a few months, you can see that the composition hardly changes. But in stressful situations or in response to physiological or dietary changes, microflora can also change, creating an imbalance in the interaction between microflora and its host. And such changes can affect human health.
Impact on health status.
Vzaimonapravlennykh connection between the gut and the brain are carried out through the endocrine, nervous, immune, and non-specific natural immunity. Intestinal microflora as an active participant in the intestinal-brain axis not only has an impact on intestinal function, but also stimulates the development of the CNS in the perinatal period and interacts with the higher nerve centers, causing depression and cognitive dysfunction in the pathology. Special role of microglia intestine. In addition to the mechanical (protective) and trophic functions for enteric neurons, glia performs neurotransmitter, immunologic, barrier and motor function in the intestine. There is a relationship between the barrier function of the gut and regulation of blood-brain barrier.
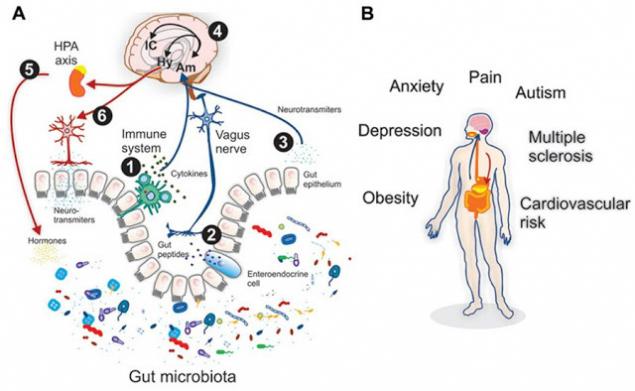
Chronic endotoxinemia (high level of toxins in the blood) as a result of dysfunction of the intestinal barrier forms an inflammatory condition in the periventricular tissue does areas of the brain with subsequent destabilization of the blood-brain barrier and the spread of inflammation to other areas of the brain, resulting in the development of neurodegeneration.
It is established that the microbiota affecting the barrier function of the mucous membrane and causes the immune and neuroendocrine response, may give direct and indirect effects on the function and even morphology of the muscular and nervous cells of the intestine. Studies have shown the links between inflammation of the mucous membrane and motor and sensory functions of the gut, the violation of its barrier function in the modification of microbiota and effects of changes in the integrity of the mucous membrane to the host. The immune response induced by pathogens has attracted increased attention of researchers, given the possible contribution of inflammation in the pathogenesis of motor dysfunction in various diseases.
Depression and microflora.
For example, it is now known that depression has an inflammatory component and many useful bacteria in the gut produce short-chain fatty acids such as butyrate, contributing to the nutrition of the cells lining the intestine to reduce inflammation. The microbiome associated with depression recently, when it was discovered that bacteria produce Oscillibacter chemical substance that acts as a natural tranquilizer that mimic the action of neurotransmitter GABA (this neurodematitis — gamma-aminobutyric acid lowers neural activity of the brain and can lead to depression). The ability of soil microbes, such as Mycobacterium Vacca (Mycobacterium vaccae), to modulate the immune system have long been known, and some researchers even suggest that this property can be used to create a vaccine against stress and depression.
In particular, Graham from University College London claims that insufficient contact with our old friends — the soil microbes, the effects of which we have been subjected throughout history, but now, in his immoderate desire for purity, nullified, is the reason for the spread of many diseases, including diabetes, arthritis and depression.
Anorexia and microflora. Researchers from the Medical school of the University of North Carolina (University of North Carolina School of Medicine) believe that this bacterial imbalance can be associated with certain psychological symptoms occurring in this disease, which, as you know, has the highest mortality rate than any other mental health condition. It is known that microbial diversity – a sign of good overall health. Previous studies also suggest that the abundance and diversity of intestinal microflora, could affect the so-called "axis of the bowel-brain". To the extent that, as in patients with anorexia nervosa improved intestinal microbiome, and increased weight and improved mood of patients, which suggests the existence of a relationship between these factors.
Anxiety, inflammation and microflora.
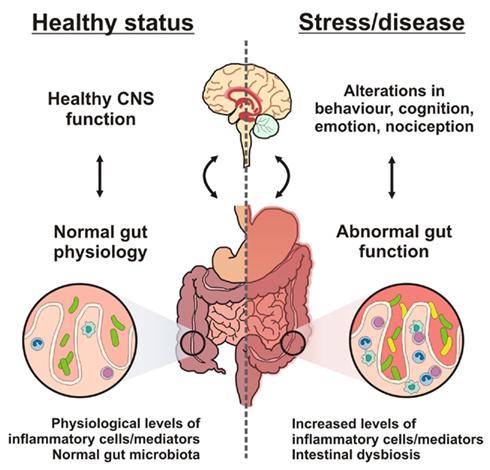
Because the intestinal microflora plays a key role in the development of immunity, we can assume that sterile mice, the inflammatory processes are always quiet. When we consider the relationship between inflammation and disturbing behavior, we can observe that the lowest anxiety is found in the same place where inflammatory processes are not strongly expressed, but a more severe inflammation leads to increased anxiety. For example, infection of mice with the parasite Trichuris muris leads to inflammation in the gut and increase levels of anxiety. In addition, chemically induced inflammation (colitis) also leads to increased anxiety. In the same studies provided the evidence that the microbiota acts as a modulator of anxiety in this behavior associated with the immune response: the report States that “treatment” probiotic culture Bifidobacterium longum reduced this anxiety. These observations suggest that the appointment of probiotics may be a promising approach in the treatment of inflammatory processes and related symptoms of “anxiety”.
A group of Mak-Master of the University began to look for answers by exploring mice. In the study in 2011, the team transplanted samples of the intestinal microflora between different strains of mice and showed that behavioral traits characteristic of a particular strain is passed along with it. Bercik says, for example, that "relatively shy" mice will demonstrate more "exploratory" behavior at transplant their microbiota seeking adventure mice. "I think it's amazing. Microbiota do determines the phenotype of the behavior of the host. The difference is obvious," says Bercik. Unpublished studies show that isolated from a person with IBS and anxiety fecal bacteria transplantation mice causes restless behavior and they, while transplanting bacteria healthy individuals this has no effect.
Stress and microflora
One of the first studies examining the relationship of stress and microflora showed that sterile mice, the stress response is too intense. And another, more recent study showed that exposure to stress of rats “in his youth” causes changes in the composition of the microflora and leads to more intense stress reactions in adulthood. Importantly, this study found that if the rats give a probiotic (Lactobacillus sp) it normalizes levels of stress hormones. Stress in the early stages of life leads to a more depressive behavior in Mature rats. Another similar study showed that if rat youngsters are vulnerable to stress, give probiotics (bacteria Bifiodo infantis), reduced symptoms of depression in adulthood.
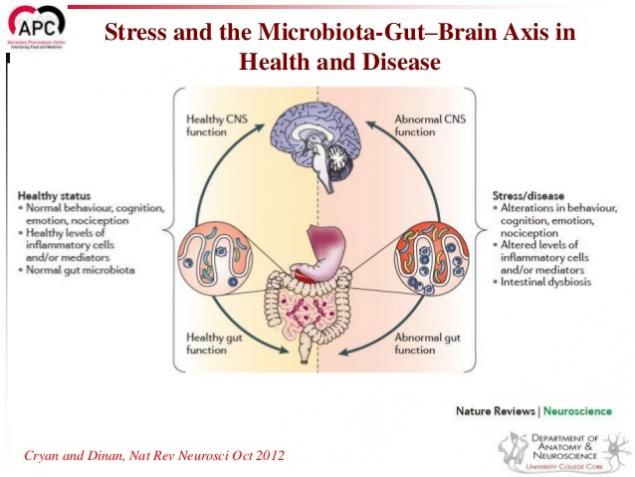
Together, these studies suggests the need to recognize the link between microflora imbalance (dysbiosis), changes in behaviour related to stress and stress reaction. Also this suggests that the use of probiotics may be effective in the treatment of symptoms associated with stress.
During the small study, which involved healthy young men, researchers from University College Cork (University College Cork), Ireland, revealed that the ingestion of probiotic preparations containing Bifidobacterium longum (B. longum), reduces the level of physiological and psychological stress and improves memory. The report on this work was presented by the head of research Dr Gerard Clarke (Gerard Clarke) at the annual meeting of the Community of neuroscience (Society for Neuroscience — SfN). He noted that the basis for its conduct began pre-clinical experiments, during which it became known that the strain B. longum has a positive effect on cognitive function in laboratory mice reduces the severity of physiological and behavioral manifestations of stress.
In this work, was attended by 22 volunteers (men, mean age 25.5 years) who within 4 weeks took the product containing the strain B. longum NCIMB 41676, and then following 4 weeks or placebo. At the beginning and at the end of each 4-week period, researchers evaluated the level of acute stress from private owners, using the cold Pressor test and measuring the level of cortisol, a stress hormone, and daily — using the Scale of perceived stress of Cohen (Cohen Perceived Stress). The condition of cognitive functions in volunteers was determined based on indicators of neurological activity and results of neuropsychological tests.
After analyzing the results, the study authors noted that the drug containing the probiotic strain B. longum NCIMB 41676, led to a decrease in cortisol levels and subjective to reduce the level of anxiety. Participants stated that while taking the drug, they felt less tense than in the beginning of the study, and their visual memory is much improved.
The researchers stressed that the new concept, considering the microbiota as a key regulator of the behavior and functioning of the brain represents a paradigm shift in neuroscience. Point medical intervention is in the axis of "microbiota — gut — brain" with priobiotics — organisms with a potentially positive impact on mental health can be seen as a new approach to the treatment of pathological conditions associated with stress. They believe that further work should be the study of the mechanisms underlying the above relationships.
Conclusion
Intestinal microflora (microbiota) – a huge population, important for healthy metabolism and brain function, and the communication between gut and brain is also through neural connections. The intestinal microflora is very important at an early age and can influence what reactions to stress will be developed in the brain
Probiotics (research on humans and animals have shown that probiotics or in other words “good bacteria”, have a positive impact on mood. Although this is a very promising open, no need to rush and think that we have already found a solution for clinical situations (disorders of conduct and mood). Of course, the microflora is an important modulator of health and must be considered a component of a complex, multi-faceted system of communication, which is necessary to establish a healthy balance for the development and healthy functioning of the brain.
But! The criterion of gut health is not only a single probiotic, namely the diversity of the microflora.
Therefore, an important normalization of food in General! Alas, a unique probiotic does not exist. What to do to improve microflora, write then. published
Author: Andrey Blueskin
P. S. And remember, only by changing their consumption — together we change the world! © Join us at Facebook , Vkontakte, Odnoklassniki
Source: www.beloveshkin.com/2015/11/nevidimaya-os-mozg-kishechnik-tvoj-mozg-i-eda.html
A so-called enteric nervous system, located between the esophagus and the intestine, consists of 100 million nerve cells. Please note: there are more of them than in the spinal cord. This is the second complex cluster of nerves in the human body after the brain. Our brain, with all its feelings, emotions and thoughts constantly communicates with "kisacanin brain." This process of communication is called "the axis brain – gut".
Remember that healthy nutrition is half of health. And healthy diet includes the impact of foods on our little intestinal friends. Remember that food is not just calories and energy. Food contains information, which she tells to your genes, turning on and off them, moment to moment, influencing their function. Food is the most powerful and fast-acting medicine that you can take to change your life. Food is not only calories. This is the information. It tells the genes what to do (and not do).
What is the axis of the gut-brain?
The axis the “gut-brain” – an imaginary line connected and one of the new horizons complex neuroscience. Gut microbiota (or microflora), which is often now called the “second genome” and the “second brain,” may influence our mood through mechanisms that scientists are only beginning to understand. And, unlike the genes that we inherit, the microflora can be changed and even grown. As soon as studies are transferred from mice not people, we are getting more understanding of the relationships of microflora our brains become visible an important connection with the mental (or emotional) health. A Japanese magnate was once asked how he will know whether to join the deal and he replied: "I swallow it, and if I like the feeling in my stomach, I enter into a transaction". Our gut with your own mind, but is continuously talking to our brain.
Digestion is a complex process, so there is nothing surprising in the fact that for its regulation there is a separate neural network. Digestive the nervous system is responsible for the processes of mechanical mixing of food in the stomach, coordinating the contraction of circular muscles and all of the sphincter throughout the bowel to ensure the progress of food, it also supports different biochemical environment and the level of acidity inside each individual section of the digestive tract, enabling the enzymes necessary conditions for their work.
Do not have to be a gastroenterologist to be aware of these reactions, or may be a more subtle feeling in my stomach that accompany emotions such as anxiety, excitement, fear or stress. For millennia, people were convinced that the gastrointestinal tract is associated with the brain and affect health. Only in the last century this relationship has been extensively studied. Two pioneers in this field was the American physician B. Robinson (published in 1907 his work titled "the Abdominal and Pelvic Brain") and his contemporary British physiologist Ivan Langley, who coined the term "gastro-intestinal nervous system".
In the early twentieth century Englishman Newport Langley calculated the number of nerve cells in the stomach and intestines — 100 million. More than in the spinal cord! There are no hemispheres, but in the presence of a branched network of neurons and supporting cells along with all sorts of impulses and signals. It has been suggested: is it possible to count such a conglomeration of nerve cells a kind of "ventral" brain?

The intestinal brain.

Recently on this subject said the Professor of neurogastroenterology Paul Enk from the University of Tubingen: "the Brain the stomach is about the same as the head. It can be represented in the form of a stocking which covers the esophagus, stomach and intestines. In the stomach and intestines of people suffering from Alzheimer's and Parkinson's, discovered the same tissue damage as in the brain. Therefore, the antidepressants like prozac that affect on the stomach."
A decade after the release of the most popular works of the "Second brain" American scientist confirms the assumption that the nervous system of the intestine is not stupid cluster nodes and tissues that executes commands of the Central nervous system, according to an old medical doctrine, and a unique network able to carry out complex processes on their own.
It is noteworthy that the gut continues to function even when there is no communication with the brain and spinal cord. Intestinal brain to decide all aspects of digestion throughout the gastrointestinal tract — from the esophagus to the intestine and rectum. They used the same tools as the "noble" brain: a whole web of neural circuits, neurotransmitters and proteins. Evolution is evidence of his insight: instead of trying to force the head of the severely strain the activity of millions of nerve cells to communicate with a remote site of the body, she chose to entrust the management of the center, located in controlled areas.
According to modern concepts, the neurotransmitters produced by the neurons of the gastrointestinal tract, are not able to get into the brain, however they are still unable to penetrate a small area of the brain where the blood-brain barrier permeability is higher, for example, in the hypothalamus. Whatever it was, nervous signals sent from the gastrointestinal tract to the brain, no doubt, affect the mood. Researchers began to decipher the ways in which gut bacteria can signal the brain. Peterson and others have shown that in adult mice microbial metabolites affect the basic physiology of the blood-brain barrier. Gut microbes break down complex carbohydrates to short chain fatty acids with the formation of a mass of effects: the fatty acid butyrate, for example, strengthen the blood-brain barrier, “tightening” the connections between cells.
Coexistence of symbiotic microflora and its media, for the most part, mutually beneficial. In particular, the presence of symbionts is essential for the functioning of our immune system, digest nutrients and other aspects of healthy physiology. Using the most modern tools to study genetics and tissues at the molecular level, scientists were able to demonstrate that in the gut are a few types of bacteria, and that symbiotic populations are characterized by great variety: you can allocate up to thousands of different species. In addition, the formation of individual microflora is constantly influenced by such factors as gender, genetics, age, type of food .
In healthy people, biological diversity is much more but at the same time, studying the microflora of these people in different moments of time (a few months, you can see that the composition hardly changes. But in stressful situations or in response to physiological or dietary changes, microflora can also change, creating an imbalance in the interaction between microflora and its host. And such changes can affect human health.
Impact on health status.
Vzaimonapravlennykh connection between the gut and the brain are carried out through the endocrine, nervous, immune, and non-specific natural immunity. Intestinal microflora as an active participant in the intestinal-brain axis not only has an impact on intestinal function, but also stimulates the development of the CNS in the perinatal period and interacts with the higher nerve centers, causing depression and cognitive dysfunction in the pathology. Special role of microglia intestine. In addition to the mechanical (protective) and trophic functions for enteric neurons, glia performs neurotransmitter, immunologic, barrier and motor function in the intestine. There is a relationship between the barrier function of the gut and regulation of blood-brain barrier.

Chronic endotoxinemia (high level of toxins in the blood) as a result of dysfunction of the intestinal barrier forms an inflammatory condition in the periventricular tissue does areas of the brain with subsequent destabilization of the blood-brain barrier and the spread of inflammation to other areas of the brain, resulting in the development of neurodegeneration.
It is established that the microbiota affecting the barrier function of the mucous membrane and causes the immune and neuroendocrine response, may give direct and indirect effects on the function and even morphology of the muscular and nervous cells of the intestine. Studies have shown the links between inflammation of the mucous membrane and motor and sensory functions of the gut, the violation of its barrier function in the modification of microbiota and effects of changes in the integrity of the mucous membrane to the host. The immune response induced by pathogens has attracted increased attention of researchers, given the possible contribution of inflammation in the pathogenesis of motor dysfunction in various diseases.
Depression and microflora.
For example, it is now known that depression has an inflammatory component and many useful bacteria in the gut produce short-chain fatty acids such as butyrate, contributing to the nutrition of the cells lining the intestine to reduce inflammation. The microbiome associated with depression recently, when it was discovered that bacteria produce Oscillibacter chemical substance that acts as a natural tranquilizer that mimic the action of neurotransmitter GABA (this neurodematitis — gamma-aminobutyric acid lowers neural activity of the brain and can lead to depression). The ability of soil microbes, such as Mycobacterium Vacca (Mycobacterium vaccae), to modulate the immune system have long been known, and some researchers even suggest that this property can be used to create a vaccine against stress and depression.
In particular, Graham from University College London claims that insufficient contact with our old friends — the soil microbes, the effects of which we have been subjected throughout history, but now, in his immoderate desire for purity, nullified, is the reason for the spread of many diseases, including diabetes, arthritis and depression.
Anorexia and microflora. Researchers from the Medical school of the University of North Carolina (University of North Carolina School of Medicine) believe that this bacterial imbalance can be associated with certain psychological symptoms occurring in this disease, which, as you know, has the highest mortality rate than any other mental health condition. It is known that microbial diversity – a sign of good overall health. Previous studies also suggest that the abundance and diversity of intestinal microflora, could affect the so-called "axis of the bowel-brain". To the extent that, as in patients with anorexia nervosa improved intestinal microbiome, and increased weight and improved mood of patients, which suggests the existence of a relationship between these factors.
Anxiety, inflammation and microflora.
Because the intestinal microflora plays a key role in the development of immunity, we can assume that sterile mice, the inflammatory processes are always quiet. When we consider the relationship between inflammation and disturbing behavior, we can observe that the lowest anxiety is found in the same place where inflammatory processes are not strongly expressed, but a more severe inflammation leads to increased anxiety. For example, infection of mice with the parasite Trichuris muris leads to inflammation in the gut and increase levels of anxiety. In addition, chemically induced inflammation (colitis) also leads to increased anxiety. In the same studies provided the evidence that the microbiota acts as a modulator of anxiety in this behavior associated with the immune response: the report States that “treatment” probiotic culture Bifidobacterium longum reduced this anxiety. These observations suggest that the appointment of probiotics may be a promising approach in the treatment of inflammatory processes and related symptoms of “anxiety”.
A group of Mak-Master of the University began to look for answers by exploring mice. In the study in 2011, the team transplanted samples of the intestinal microflora between different strains of mice and showed that behavioral traits characteristic of a particular strain is passed along with it. Bercik says, for example, that "relatively shy" mice will demonstrate more "exploratory" behavior at transplant their microbiota seeking adventure mice. "I think it's amazing. Microbiota do determines the phenotype of the behavior of the host. The difference is obvious," says Bercik. Unpublished studies show that isolated from a person with IBS and anxiety fecal bacteria transplantation mice causes restless behavior and they, while transplanting bacteria healthy individuals this has no effect.
Stress and microflora
One of the first studies examining the relationship of stress and microflora showed that sterile mice, the stress response is too intense. And another, more recent study showed that exposure to stress of rats “in his youth” causes changes in the composition of the microflora and leads to more intense stress reactions in adulthood. Importantly, this study found that if the rats give a probiotic (Lactobacillus sp) it normalizes levels of stress hormones. Stress in the early stages of life leads to a more depressive behavior in Mature rats. Another similar study showed that if rat youngsters are vulnerable to stress, give probiotics (bacteria Bifiodo infantis), reduced symptoms of depression in adulthood.

Together, these studies suggests the need to recognize the link between microflora imbalance (dysbiosis), changes in behaviour related to stress and stress reaction. Also this suggests that the use of probiotics may be effective in the treatment of symptoms associated with stress.
During the small study, which involved healthy young men, researchers from University College Cork (University College Cork), Ireland, revealed that the ingestion of probiotic preparations containing Bifidobacterium longum (B. longum), reduces the level of physiological and psychological stress and improves memory. The report on this work was presented by the head of research Dr Gerard Clarke (Gerard Clarke) at the annual meeting of the Community of neuroscience (Society for Neuroscience — SfN). He noted that the basis for its conduct began pre-clinical experiments, during which it became known that the strain B. longum has a positive effect on cognitive function in laboratory mice reduces the severity of physiological and behavioral manifestations of stress.

In this work, was attended by 22 volunteers (men, mean age 25.5 years) who within 4 weeks took the product containing the strain B. longum NCIMB 41676, and then following 4 weeks or placebo. At the beginning and at the end of each 4-week period, researchers evaluated the level of acute stress from private owners, using the cold Pressor test and measuring the level of cortisol, a stress hormone, and daily — using the Scale of perceived stress of Cohen (Cohen Perceived Stress). The condition of cognitive functions in volunteers was determined based on indicators of neurological activity and results of neuropsychological tests.
After analyzing the results, the study authors noted that the drug containing the probiotic strain B. longum NCIMB 41676, led to a decrease in cortisol levels and subjective to reduce the level of anxiety. Participants stated that while taking the drug, they felt less tense than in the beginning of the study, and their visual memory is much improved.
The researchers stressed that the new concept, considering the microbiota as a key regulator of the behavior and functioning of the brain represents a paradigm shift in neuroscience. Point medical intervention is in the axis of "microbiota — gut — brain" with priobiotics — organisms with a potentially positive impact on mental health can be seen as a new approach to the treatment of pathological conditions associated with stress. They believe that further work should be the study of the mechanisms underlying the above relationships.

Conclusion
Intestinal microflora (microbiota) – a huge population, important for healthy metabolism and brain function, and the communication between gut and brain is also through neural connections. The intestinal microflora is very important at an early age and can influence what reactions to stress will be developed in the brain
Probiotics (research on humans and animals have shown that probiotics or in other words “good bacteria”, have a positive impact on mood. Although this is a very promising open, no need to rush and think that we have already found a solution for clinical situations (disorders of conduct and mood). Of course, the microflora is an important modulator of health and must be considered a component of a complex, multi-faceted system of communication, which is necessary to establish a healthy balance for the development and healthy functioning of the brain.
But! The criterion of gut health is not only a single probiotic, namely the diversity of the microflora.
Therefore, an important normalization of food in General! Alas, a unique probiotic does not exist. What to do to improve microflora, write then. published
Author: Andrey Blueskin
P. S. And remember, only by changing their consumption — together we change the world! © Join us at Facebook , Vkontakte, Odnoklassniki
Source: www.beloveshkin.com/2015/11/nevidimaya-os-mozg-kishechnik-tvoj-mozg-i-eda.html