448
Glycation and its products: Garbage inside your body
In the process of cooking, nutrients do not remain passive, but actively interact with each other. Of particular importance among these processes is the interaction of sugars and proteins, the so-called glycation, or non-enzymatic glycosylation, which is also the Maillard reaction.
This reaction can occur in different forms: both when cooking and in our body when glucose levels increase. At the end of this and a number of other reactions, the formation of so-called “final glycation products” occurs, which are cellular debris, slags that clog the cell and rebuild all its work.
The topic is large, so first we will analyze the glycation reaction itself and the conditions predisposing to it. Then we learn what metabolic memory is and how AGE products (also known as Advanced Glycosylation End-products) affect our metabolism. And, of course, what to do with it both in the process of cooking and inside our body. !
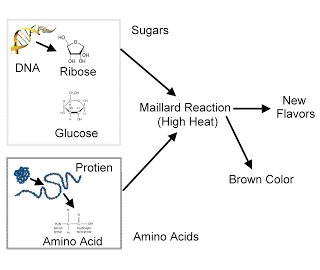
Mayar's reaction stages.
Maillard reaction (saccharoamine condensation reaction) is a chemical reaction between an amino acid and a sugar that usually occurs when heated. An example of this reaction is frying meat or baking bread, when in the process of heating a food product there is a typical smell, color and taste of cooked food. These changes are caused by the formation of Maillard reaction products.
Let us not confuse glycation and glycosylation. Glycoproteins are important biochemical compounds formed by enzymes and performing specific functions. Examples of such glycoproteins are hyaluronic acid and chondroitin sulfate. When sugar reacts with proteins without enzymes, the result is AGEs, which are harmful to the body.
Thus, if glycosylation is a normal, genetically controlled mechanism involving enzymes, then glycation is not an enzymatic or gene-programmed process that is not beneficial.
Glucose in its usual form – D-glucopyranose – is a rather inert molecule, which is the most important source of nutrition for the cell. It is the only sugar that circulates in excess in the body.
Although relatively harmless, it can become dangerous when transformed into AGE by a complex, random process common to animals and plants. This process of darkening does not require the participation of enzymes, it depends only on the temperature and abundance of reactive components.
In the first step of this process, glucose and other simple sugars react with proteins, and then, combining with amino acids and other components, trigger a further reaction. For example, lysine glycation produces fructose-lysine, which can be broken down into constituents such as carboxymethyllysine (CML) and pentosidine. As a result, elastin and collagen lose their ability to separate and new proteins are formed to replace them.
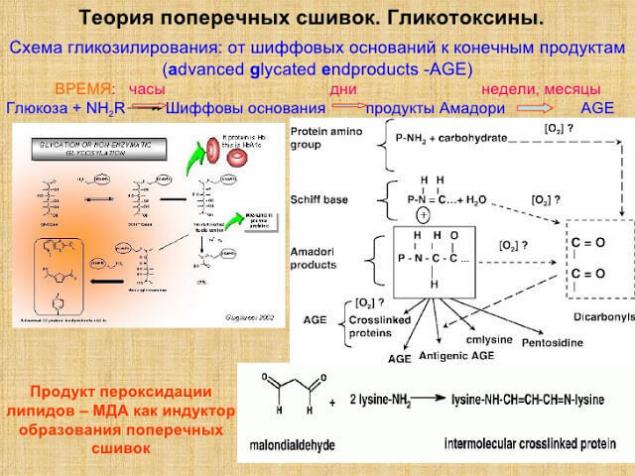
According to Mailard’s theory, protein crosslinks are formed as a result of the damaging action of monosacchars. This process is multi-step.
It begins with reversible glycation - the reduced sugar (glucose, fructose, ribose, etc.) joins the terminal α-amino group of protein. This happens spontaneously, without the participation of enzymes.
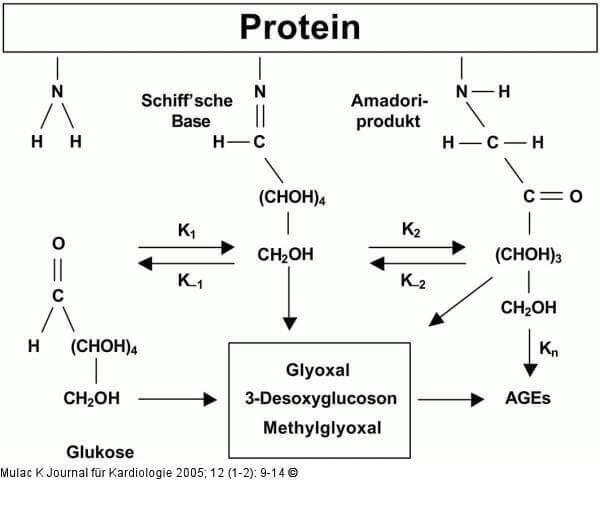
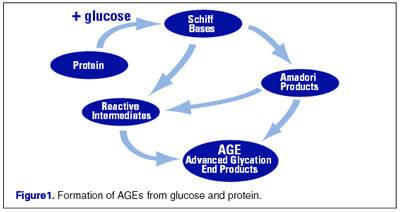
In fact, there is a typical condensation reaction known from organic chemistry – a reaction between an aldehyde group and an α-amino group, which results in the formation of Schiff bases. In this case, substances formed by the primary condensation of protein and reduced sugar are called Amadori products.
In the future, Amadori products are subjected to various, mostly irreversible, modifications (oxidation, condensation, structural adjustments, etc.). As a result, a fairly diverse group of substances is formed, called Advanced Glycosylation End-products (AGE). AGEs slowly accumulate in tissues and have many negative effects.
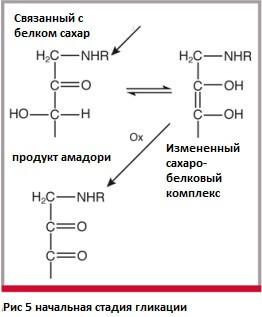
The glycation reaction includes several stages: stage one is condensation.
The Maillard reaction begins when the aldose carbonyl group (HC=O) combines with a free amino group of an amino acid (-NH2), usually a protein or peptide, resulting in an N-substituted aldosylamine. Simply put, sugar combines with amino acid.
In general, it is a sugar dehydration reaction to form water, and the condensation product rapidly loses water as it becomes a Schiff base. Schiff bases are characterized by a double bond of carbon with nitrogen, and nitrogen in them is associated with an aryl or alkyl group (H-C=N-R).
Then the base of Schiff acquires a ring structure. This rearrangement of the structure called the Amadori rearrangement forms ketosamine in the process of changing the molecular structure around the oxygen atom. If we take glucose as an aldose, and glycerol as an amino acid, then as a result of rearrangement of Amadori we get 1-amino-1-dioxy-2-fructose or monofructosaglycerin. Amadori rearrangement is a key step in the formation of intermediate components involved in the darkening reaction.
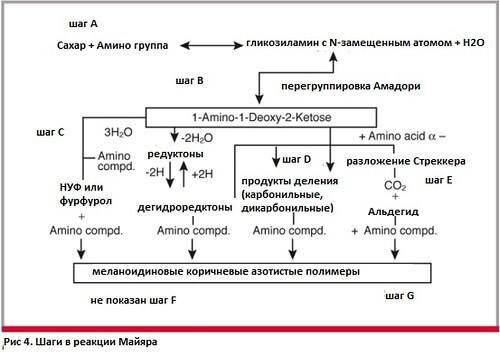
Stage two - decay, decomposition
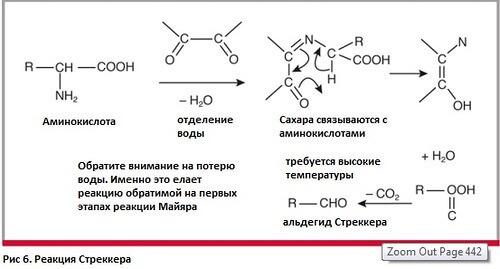
Further, the product obtained from the Amadori reaction can be broken down in three different ways, depending on the conditions. In the decomposition of Strecker (Fig. 6), amino acids undergo oxidative decay under the action of carbonyl components that appear as a result of the decomposition of ketosamines.
In this decomposition reaction, amino acids emerge from the Schiff bases and then undergo a process of decarboxylation catalyzed by acids. Schiff's new bases are easily hydrolyzed to amines and aldehydes. As a result of the decomposition of Stacker, CO2 is released and a transamination reaction occurs, which combines nitrogen with melanoids. The formed aldehydes contribute to the appearance of aroma and participate in the formation of melanoidins.
Stage three glycation – polymerization and darkening
This stage is characterized by the formation of dark pigment and the smell of fried. The formation of melanoidins is the result of polymerization of highly reactive components at a late stage of the Maillard reaction.
This can be characterized by not very pleasant or sharp smells: there is a smell of burnt, rotten, the smell of onions, solvent or cabbage. There may be pleasant aromas - malt, toasted bread crust, caramel or coffee. The chemical composition of these components is not well known.
End products of glycation (AGE).
At the end of all these transformations, the “end products of glycation”, Advanced Glycosylation End-products (AGE), are formed, which have an adverse effect on metabolism. Of course, among these compounds there are relatively harmless, and there are also very toxic. For toxic glycation end products, there is a name – glycotoxins.
The Maillard reaction doesn’t just happen when cooking. This reaction between proteins and sugars (the so-called glycation) takes place in a living organism. Under normal conditions, the reaction rate is so low that its products can be removed.
However, with a sharp increase in blood sugar in diabetes, the reaction is significantly accelerated, the products accumulate and can cause numerous disorders (for example, hyperlipidemia). This is especially pronounced in the blood, where the level of damaged proteins increases sharply (for example, the concentration of glycosylated hemoglobin is an indicator of the degree of diabetes).
The accumulation of altered proteins in the lens causes severe visual impairment in patients with diabetes. The accumulation of some late products of the Maillard reaction, as well as the oxidation products that occur with age, leads to age-related changes in tissues.
The most common late reaction product is carboxymethyllysine, a lysine derivative. Carboxymethyllysine in the composition of proteins serves as a biomarker of general oxidative stress of the body. It accumulates with age in tissues, such as skin collagen, and is elevated in diabetes.
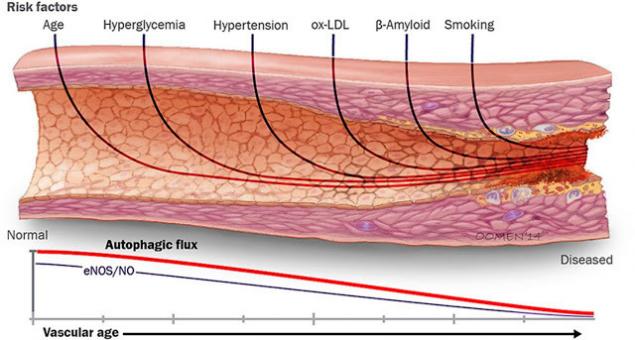
In the form of AGE, glucose becomes a kind of molecular glue that makes blood vessels inelastic and stenous. It causes inflammation, which in turn leads to hypertrophy of the smooth vascular muscles and extracellular matrix. These processes contribute to atherogenesis (the development of atherosclerosis), which occurs at a higher rate in diabetics due to elevated glucose levels.
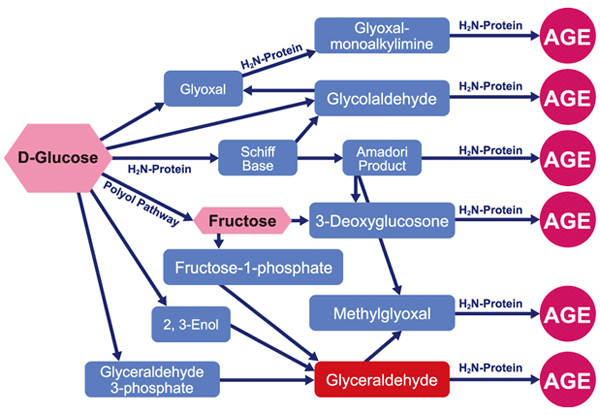
The two most common carbonyl end products of glycation in the body are methylglyoxal and glyoxal. Carbonyls are by-products of the first stage of the Maillard reaction and are reactive compounds. Methylglyoxal and glyoxal can be obtained from glucose without undergoing a full Maillard reaction cycle.
Due to its reactivity, methylglyoxal plays an important role in the formation of late glycation products during the Maillard reaction. Moreover, it is considered the most important of the glycating reagents (i.e. covalently binding to amino groups of proteins, such as glucose, galactose, etc.), leading to impaired protein function in diabetes and aging.
Biomolecule modification.
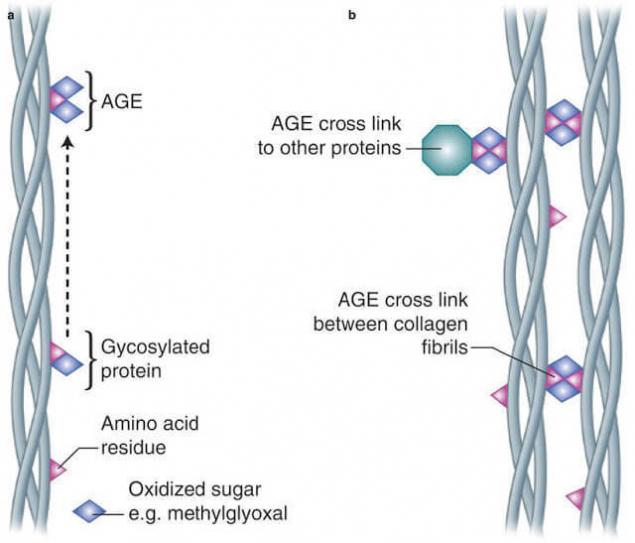
Under the action of AGE, various biomolecules are modified. This, of course, leads to a deterioration in the structure of the various organs. One of the main proteins of the skin, as well as tendons, ligaments and bones, is collagen. It is a little 20-30% of the weight of the whole body. And it is the changes that occur with it that are responsible for the appearance of wrinkles, a decrease in skin elasticity, etc. In the normal state between the triplets of tropocollagen, there are crosslinks, i.e. covalent chemical bonds that give the collagen fibers the necessary mechanical properties.
However, with age, the number of crosslinks between tropocollagen units increases. This process, involving such a common substance in the tissues as glucose, occurs more intensively in patients with diabetes. It was the study of the latter that shed light on the collagen theory of aging.
The carbonyl group of reducing sugars, including such a common substance in our body as glucose, reacts with free terminal amino groups of the amino acid lysine, which is very rich in collagen.
This trivial transformation is known to us chemists as the nucleophilic addition of the carbonyl group, and is called the Maillard reaction. The product of this reaction with the beautiful name of the base of Schiff later undergoes more complex transformations with even more mysterious names, for example, the rearrangement of Amadori.
Amadori product as a result of proton migration, cyclization and numerous dehydration turns into an activated carbonyl compound, lovingly attaching the residue of arginine of the neighboring tropokollagen chain, forming, for example, glucosepane crosslinking.
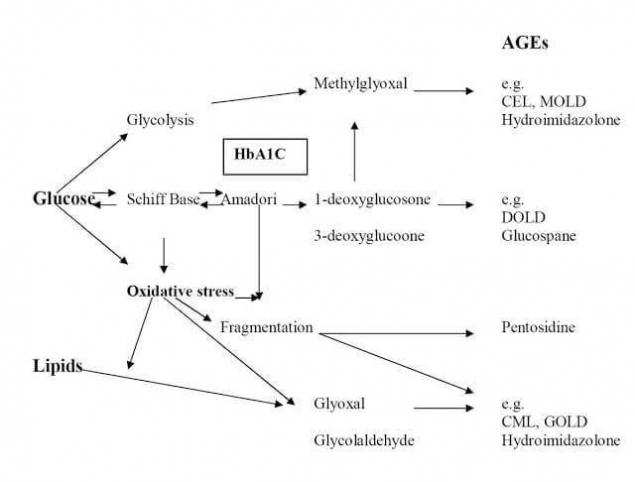
Simplified scheme of the complex Maillard reaction and formation of some advanced glycation endproducts (AGEs) in vivo. CEL = carboxyethyllysine; MOLD = methylglyoxal lysine dimer; DOLD, 3-deoxyglucosone lysine dimer; CML, carboxymethyllysine; GOLD, glyoxal lysine dimer. Redrawn with permission from Monnier VM, Arch Biochem Biophys. 2003;419:1-15.
By the way, similar processes, however, occurring at high temperatures, cause the formation of a brown crust on bakery products. Does this brown crust remind you of anything? What does the increase in the number of crosslinks between collagen molecules lead to? The first consequence of this phenomenon, as you might guess, is a change in the mechanical properties of tissues. Naturally, this applies to the skin, which with age loses its elasticity, i.e. becomes more rigid.
Imagine that you are simultaneously stretching with both hands 5 rubber harnesses. Now imagine that in several places these harnesses are connected to each other by nodes. The sections of the harnesses between the nodes will be stretched to a much lesser extent.
The same thing happens with the skin. Naturally, the situation is aggravated by the fact that the collagen content in the skin decreases with age, as the activity of enzymes involved in its synthesis decreases. But even if this did not happen, the situation would still not be much corrected, since it is much more difficult to split collagen with frequent crosslinks to replace it with a new one than with rare ones.
Increasing the number of bonds in collagen reduces its elasticity. Such a change at the molecular level can cause thickening of the basal membrane, for example, in the mesangial matrix of the kidneys, and lead to renal failure in diabetes, as well as cause age-related decline in kidney function.
This mechanism is believed to play a role in narrowing arteries, reducing vascular blood flow and reducing tendon flexibility. It is shown that in the collagen of the skin of short- and long-lived animal species, the level of the glycosylation marker pentosidine is inversely proportional to the species maximum life expectancy. The level of glycosylation end products is associated with nerve damage and a tendency to form skin lesions that respond poorly to treatment.
Blood vessel damage.
The process of glycation of collagen triggers a number of formations in those organs where it plays an important structural role: the skin, lens, kidneys, vessels, intervertebral discs, cartilage, etc.
Arteriosclerosis is initiated by prolonged hyperglycemia, reactions of chemical glycation of collagen chains and elastin of loose connective tissue as a result of the chemical effect of glucose and its metabolites - glycotoxins (glyoxal and methylglyoxal), the formation of cross-links between collagen and elastin fibers.
Unlike arteriosclerosis in atheromatosis - the main manifestation of atherosclerosis - the defeat of arteries of elastic type occurs due to the accumulation in the intima of lipids - cholesterol-esterified essential unsaturated and polyene fatty acids, the formation of plaques in the localization of sedentary macrophages in intima, foci of necrosis and calcinosis; atheromatosis does not affect collagenous and elastic structures in the artery wall.
Arteriosclerosis and atheromatosis as a manifestation of atherosclerosis are two independent pathological processes in the wall of elastic arteries. Arteriolosclerosis is a consequence of the glycation of collagen and elastin chains in the wall of muscle-type arterioles, poststarterioles, in the endothelium and pericytes of exchange capillaries. Microangiopathy initiates only the processes of glycation and the action of glycotoxins, since there is no intima in muscle-type arterioles, which is a local interstitial tissue for collecting and recycling biological “junk” from the blood, from the intravascular pool of the intercellular environment.
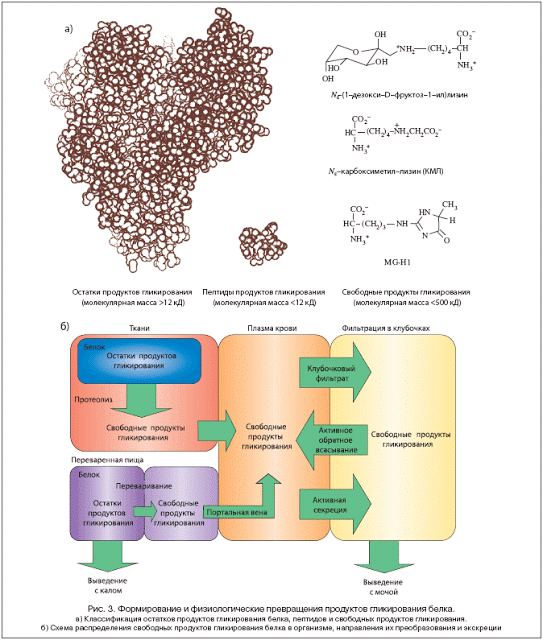
Other proteins and DNA.
Nucleic acids and proteins can be modified by attaching sugars to their free amino groups, leading to structural and functional rearrangement of molecules. Nucleotides and DNA also undergo non-enzymatic glycosylation, which leads to mutations due to direct DNA damage and inactivation of recombination error repair systems, and also causes increased chromosome fragility. Non-enzymatic glycosation of biologically important molecules is becoming an increasingly important area in the study of diabetes and the normal aging process.
First of all, long-lived proteins suffer, that is, they glycate: hemoglobins, albumins, collagen, crystallins, low-density lipoproteins. The consequences are most unpleasant. For example, glycation of proteins of the erythrocyte membrane makes it less elastic, more rigid, as a result of which the blood supply to tissues deteriorates.
Due to the glycation of crystallines, the lens becomes cloudy and, as a result, cataracts develop. Modified proteins in this way we can detect, and therefore they serve as markers of atherosclerosis, diabetes, neurodegenerative diseases. Doctors and diabetics are familiar with one specific end product of glycation - A1c.
It is formed as a result of the Amadori reaction by attaching glucose to the β chain of normal hemoglobin. Today, one of the fractions of glycated hemoglobin (HbA1c) is among the main biochemical markers of diabetes and cardiovascular disease. A 1% reduction in HbA1c reduces the risk of any diabetes complications by 20%.
Amino acid damage.
The disadvantages of glycation should be attributed to the fact that the Mailar reaction reduces the biological value of proteins, since amino acids, especially lysine, threonine, arginine and methionine, which are most often lacking in the body, after combining with sugars become inaccessible to digestive enzymes and, therefore, are not absorbed.
Author: Andrey Beloveskin
Teeth hurt in hesitant people or what the problems with the teeth say
11 tips about having a baby that no one gave you
Source: www.beloveshkin.com/2016/05/glikirovanie-i-ego-produkty-musor-vnutri-vashego-tela.html
This reaction can occur in different forms: both when cooking and in our body when glucose levels increase. At the end of this and a number of other reactions, the formation of so-called “final glycation products” occurs, which are cellular debris, slags that clog the cell and rebuild all its work.
The topic is large, so first we will analyze the glycation reaction itself and the conditions predisposing to it. Then we learn what metabolic memory is and how AGE products (also known as Advanced Glycosylation End-products) affect our metabolism. And, of course, what to do with it both in the process of cooking and inside our body. !

Mayar's reaction stages.
Maillard reaction (saccharoamine condensation reaction) is a chemical reaction between an amino acid and a sugar that usually occurs when heated. An example of this reaction is frying meat or baking bread, when in the process of heating a food product there is a typical smell, color and taste of cooked food. These changes are caused by the formation of Maillard reaction products.
Let us not confuse glycation and glycosylation. Glycoproteins are important biochemical compounds formed by enzymes and performing specific functions. Examples of such glycoproteins are hyaluronic acid and chondroitin sulfate. When sugar reacts with proteins without enzymes, the result is AGEs, which are harmful to the body.
Thus, if glycosylation is a normal, genetically controlled mechanism involving enzymes, then glycation is not an enzymatic or gene-programmed process that is not beneficial.
Glucose in its usual form – D-glucopyranose – is a rather inert molecule, which is the most important source of nutrition for the cell. It is the only sugar that circulates in excess in the body.
Although relatively harmless, it can become dangerous when transformed into AGE by a complex, random process common to animals and plants. This process of darkening does not require the participation of enzymes, it depends only on the temperature and abundance of reactive components.
In the first step of this process, glucose and other simple sugars react with proteins, and then, combining with amino acids and other components, trigger a further reaction. For example, lysine glycation produces fructose-lysine, which can be broken down into constituents such as carboxymethyllysine (CML) and pentosidine. As a result, elastin and collagen lose their ability to separate and new proteins are formed to replace them.

According to Mailard’s theory, protein crosslinks are formed as a result of the damaging action of monosacchars. This process is multi-step.
It begins with reversible glycation - the reduced sugar (glucose, fructose, ribose, etc.) joins the terminal α-amino group of protein. This happens spontaneously, without the participation of enzymes.
In fact, there is a typical condensation reaction known from organic chemistry – a reaction between an aldehyde group and an α-amino group, which results in the formation of Schiff bases. In this case, substances formed by the primary condensation of protein and reduced sugar are called Amadori products.
In the future, Amadori products are subjected to various, mostly irreversible, modifications (oxidation, condensation, structural adjustments, etc.). As a result, a fairly diverse group of substances is formed, called Advanced Glycosylation End-products (AGE). AGEs slowly accumulate in tissues and have many negative effects.

The glycation reaction includes several stages: stage one is condensation.
The Maillard reaction begins when the aldose carbonyl group (HC=O) combines with a free amino group of an amino acid (-NH2), usually a protein or peptide, resulting in an N-substituted aldosylamine. Simply put, sugar combines with amino acid.
In general, it is a sugar dehydration reaction to form water, and the condensation product rapidly loses water as it becomes a Schiff base. Schiff bases are characterized by a double bond of carbon with nitrogen, and nitrogen in them is associated with an aryl or alkyl group (H-C=N-R).
Then the base of Schiff acquires a ring structure. This rearrangement of the structure called the Amadori rearrangement forms ketosamine in the process of changing the molecular structure around the oxygen atom. If we take glucose as an aldose, and glycerol as an amino acid, then as a result of rearrangement of Amadori we get 1-amino-1-dioxy-2-fructose or monofructosaglycerin. Amadori rearrangement is a key step in the formation of intermediate components involved in the darkening reaction.

Stage two - decay, decomposition
Further, the product obtained from the Amadori reaction can be broken down in three different ways, depending on the conditions. In the decomposition of Strecker (Fig. 6), amino acids undergo oxidative decay under the action of carbonyl components that appear as a result of the decomposition of ketosamines.
In this decomposition reaction, amino acids emerge from the Schiff bases and then undergo a process of decarboxylation catalyzed by acids. Schiff's new bases are easily hydrolyzed to amines and aldehydes. As a result of the decomposition of Stacker, CO2 is released and a transamination reaction occurs, which combines nitrogen with melanoids. The formed aldehydes contribute to the appearance of aroma and participate in the formation of melanoidins.

Stage three glycation – polymerization and darkening
This stage is characterized by the formation of dark pigment and the smell of fried. The formation of melanoidins is the result of polymerization of highly reactive components at a late stage of the Maillard reaction.
This can be characterized by not very pleasant or sharp smells: there is a smell of burnt, rotten, the smell of onions, solvent or cabbage. There may be pleasant aromas - malt, toasted bread crust, caramel or coffee. The chemical composition of these components is not well known.

End products of glycation (AGE).
At the end of all these transformations, the “end products of glycation”, Advanced Glycosylation End-products (AGE), are formed, which have an adverse effect on metabolism. Of course, among these compounds there are relatively harmless, and there are also very toxic. For toxic glycation end products, there is a name – glycotoxins.
The Maillard reaction doesn’t just happen when cooking. This reaction between proteins and sugars (the so-called glycation) takes place in a living organism. Under normal conditions, the reaction rate is so low that its products can be removed.
However, with a sharp increase in blood sugar in diabetes, the reaction is significantly accelerated, the products accumulate and can cause numerous disorders (for example, hyperlipidemia). This is especially pronounced in the blood, where the level of damaged proteins increases sharply (for example, the concentration of glycosylated hemoglobin is an indicator of the degree of diabetes).

The accumulation of altered proteins in the lens causes severe visual impairment in patients with diabetes. The accumulation of some late products of the Maillard reaction, as well as the oxidation products that occur with age, leads to age-related changes in tissues.
The most common late reaction product is carboxymethyllysine, a lysine derivative. Carboxymethyllysine in the composition of proteins serves as a biomarker of general oxidative stress of the body. It accumulates with age in tissues, such as skin collagen, and is elevated in diabetes.
In the form of AGE, glucose becomes a kind of molecular glue that makes blood vessels inelastic and stenous. It causes inflammation, which in turn leads to hypertrophy of the smooth vascular muscles and extracellular matrix. These processes contribute to atherogenesis (the development of atherosclerosis), which occurs at a higher rate in diabetics due to elevated glucose levels.
The two most common carbonyl end products of glycation in the body are methylglyoxal and glyoxal. Carbonyls are by-products of the first stage of the Maillard reaction and are reactive compounds. Methylglyoxal and glyoxal can be obtained from glucose without undergoing a full Maillard reaction cycle.
Due to its reactivity, methylglyoxal plays an important role in the formation of late glycation products during the Maillard reaction. Moreover, it is considered the most important of the glycating reagents (i.e. covalently binding to amino groups of proteins, such as glucose, galactose, etc.), leading to impaired protein function in diabetes and aging.

Biomolecule modification.
Under the action of AGE, various biomolecules are modified. This, of course, leads to a deterioration in the structure of the various organs. One of the main proteins of the skin, as well as tendons, ligaments and bones, is collagen. It is a little 20-30% of the weight of the whole body. And it is the changes that occur with it that are responsible for the appearance of wrinkles, a decrease in skin elasticity, etc. In the normal state between the triplets of tropocollagen, there are crosslinks, i.e. covalent chemical bonds that give the collagen fibers the necessary mechanical properties.
However, with age, the number of crosslinks between tropocollagen units increases. This process, involving such a common substance in the tissues as glucose, occurs more intensively in patients with diabetes. It was the study of the latter that shed light on the collagen theory of aging.
The carbonyl group of reducing sugars, including such a common substance in our body as glucose, reacts with free terminal amino groups of the amino acid lysine, which is very rich in collagen.
This trivial transformation is known to us chemists as the nucleophilic addition of the carbonyl group, and is called the Maillard reaction. The product of this reaction with the beautiful name of the base of Schiff later undergoes more complex transformations with even more mysterious names, for example, the rearrangement of Amadori.
Amadori product as a result of proton migration, cyclization and numerous dehydration turns into an activated carbonyl compound, lovingly attaching the residue of arginine of the neighboring tropokollagen chain, forming, for example, glucosepane crosslinking.

Simplified scheme of the complex Maillard reaction and formation of some advanced glycation endproducts (AGEs) in vivo. CEL = carboxyethyllysine; MOLD = methylglyoxal lysine dimer; DOLD, 3-deoxyglucosone lysine dimer; CML, carboxymethyllysine; GOLD, glyoxal lysine dimer. Redrawn with permission from Monnier VM, Arch Biochem Biophys. 2003;419:1-15.
By the way, similar processes, however, occurring at high temperatures, cause the formation of a brown crust on bakery products. Does this brown crust remind you of anything? What does the increase in the number of crosslinks between collagen molecules lead to? The first consequence of this phenomenon, as you might guess, is a change in the mechanical properties of tissues. Naturally, this applies to the skin, which with age loses its elasticity, i.e. becomes more rigid.
Imagine that you are simultaneously stretching with both hands 5 rubber harnesses. Now imagine that in several places these harnesses are connected to each other by nodes. The sections of the harnesses between the nodes will be stretched to a much lesser extent.
The same thing happens with the skin. Naturally, the situation is aggravated by the fact that the collagen content in the skin decreases with age, as the activity of enzymes involved in its synthesis decreases. But even if this did not happen, the situation would still not be much corrected, since it is much more difficult to split collagen with frequent crosslinks to replace it with a new one than with rare ones.
Increasing the number of bonds in collagen reduces its elasticity. Such a change at the molecular level can cause thickening of the basal membrane, for example, in the mesangial matrix of the kidneys, and lead to renal failure in diabetes, as well as cause age-related decline in kidney function.
This mechanism is believed to play a role in narrowing arteries, reducing vascular blood flow and reducing tendon flexibility. It is shown that in the collagen of the skin of short- and long-lived animal species, the level of the glycosylation marker pentosidine is inversely proportional to the species maximum life expectancy. The level of glycosylation end products is associated with nerve damage and a tendency to form skin lesions that respond poorly to treatment.

Blood vessel damage.
The process of glycation of collagen triggers a number of formations in those organs where it plays an important structural role: the skin, lens, kidneys, vessels, intervertebral discs, cartilage, etc.
Arteriosclerosis is initiated by prolonged hyperglycemia, reactions of chemical glycation of collagen chains and elastin of loose connective tissue as a result of the chemical effect of glucose and its metabolites - glycotoxins (glyoxal and methylglyoxal), the formation of cross-links between collagen and elastin fibers.
Unlike arteriosclerosis in atheromatosis - the main manifestation of atherosclerosis - the defeat of arteries of elastic type occurs due to the accumulation in the intima of lipids - cholesterol-esterified essential unsaturated and polyene fatty acids, the formation of plaques in the localization of sedentary macrophages in intima, foci of necrosis and calcinosis; atheromatosis does not affect collagenous and elastic structures in the artery wall.
Arteriosclerosis and atheromatosis as a manifestation of atherosclerosis are two independent pathological processes in the wall of elastic arteries. Arteriolosclerosis is a consequence of the glycation of collagen and elastin chains in the wall of muscle-type arterioles, poststarterioles, in the endothelium and pericytes of exchange capillaries. Microangiopathy initiates only the processes of glycation and the action of glycotoxins, since there is no intima in muscle-type arterioles, which is a local interstitial tissue for collecting and recycling biological “junk” from the blood, from the intravascular pool of the intercellular environment.

Other proteins and DNA.
Nucleic acids and proteins can be modified by attaching sugars to their free amino groups, leading to structural and functional rearrangement of molecules. Nucleotides and DNA also undergo non-enzymatic glycosylation, which leads to mutations due to direct DNA damage and inactivation of recombination error repair systems, and also causes increased chromosome fragility. Non-enzymatic glycosation of biologically important molecules is becoming an increasingly important area in the study of diabetes and the normal aging process.
First of all, long-lived proteins suffer, that is, they glycate: hemoglobins, albumins, collagen, crystallins, low-density lipoproteins. The consequences are most unpleasant. For example, glycation of proteins of the erythrocyte membrane makes it less elastic, more rigid, as a result of which the blood supply to tissues deteriorates.
Due to the glycation of crystallines, the lens becomes cloudy and, as a result, cataracts develop. Modified proteins in this way we can detect, and therefore they serve as markers of atherosclerosis, diabetes, neurodegenerative diseases. Doctors and diabetics are familiar with one specific end product of glycation - A1c.
It is formed as a result of the Amadori reaction by attaching glucose to the β chain of normal hemoglobin. Today, one of the fractions of glycated hemoglobin (HbA1c) is among the main biochemical markers of diabetes and cardiovascular disease. A 1% reduction in HbA1c reduces the risk of any diabetes complications by 20%.


Amino acid damage.
The disadvantages of glycation should be attributed to the fact that the Mailar reaction reduces the biological value of proteins, since amino acids, especially lysine, threonine, arginine and methionine, which are most often lacking in the body, after combining with sugars become inaccessible to digestive enzymes and, therefore, are not absorbed.
Author: Andrey Beloveskin
Teeth hurt in hesitant people or what the problems with the teeth say
11 tips about having a baby that no one gave you
Source: www.beloveshkin.com/2016/05/glikirovanie-i-ego-produkty-musor-vnutri-vashego-tela.html
What to do if a child against a loved one
About destiny: How people try to compensate for the lack of love, joy, warmth and support