455
IMPORTANTLY! The oxidation of the body: the high price of free radicals
Imagine you take a wonderful red Apple from the bowl on the table. You cut it into thin slices. Then the phone rings, then bring the mail... Two hours later you go back to his Apple. Left on the plate the slices dark. They have been attacked and damaged by oxygen molecules of the air, the same molecules that corrode the metal of the car.
As the oxygen is doing it "dirty work", we say that an Apple and the car is oxidized.
Seventy four million six hundred ninety eight thousand nine hundred fourteen
The molecules that perform the function of oxidation, was called oxidants. Let's imagine that you left on the kitchen a warm pack of butter for another week. When you start to use it, it is probably the smell or the taste that the oil provarlo. This is another case of oxidant. The medical term for the oil or fat is a lipid. Scientists call the process of turning fresh fat rancid "oxidation of lipids in lipid peroxidation connection". Unfortunately, every cell of the body contains fat, which is gerontologist Dr. R. Welford calls "a private body butter" which at some time becomes rancid. The result can be "benign" — just faded skin, bad when it is lethal – cancer. The degree of destruction of an organism depends on many factors, including duration of response and the place where the act of "enemy". It also depends on genetic predisposition, the type of cellular nutrition, health and level of emotional stress of the individual. When a violation occurs in the blood vessels, it can cause disease of the capillaries. When it affects the DNA in the genetic information contained inside each cell nucleus, there may be defects or cancer. When the damaged lipids inside the lens of the eye, developed cataracts.
Thus, the oxygen earned the title of "universal oxidant" and I think the list of diseases and conditions caused by his action, becomes every day longer.
Charged free radicals
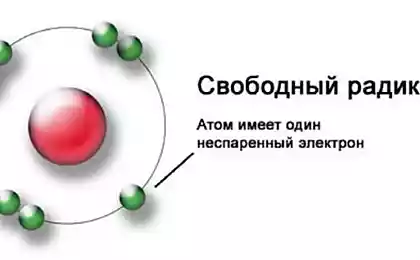
Oxidants are often free radicals. Free from what? Chemists are called radicals, tiny particles that maintain their uniqueness. In the body free radical "free" because it loses an electron.
Maybe you have seen the picture of the atom with something orbiting the nucleus? This something is an electron.
Molecules are stable when they have equal pairs of electrons (paired electrons). When a molecule loses an electron it becomes a free radical and hard and unintelligible "steals" the missing electron. Unfortunately, theft victim also becomes a free radical and immediately begins the search for his electron to the next theft, creating another free radical.
Eight million eight hundred six thousand two hundred twelve
Formed a cascade of electronic theft, and it leads to the destruction of the tissues of the body. In addition, this destructive chain reaction creates new compounds which also make a mess. Since pickpocket is the best place in the crowd, free radicals also prefer a region where accumulates a large number of electrons.
Free radicals are especially partial to polyunsaturated fatty acids, which make up about half the fat content of the membrane surrounding every cell in your body.
Where do free radicals
Free radicals are born in the process of cellular metabolism, which is in regular daily elimination of various defects, the intake of nutrients, energy production, reproduction, disposal of waste remaining after the implementation of all other functions.
Free radicals are formed also in the use of alcohol, cured meats; they appear during the artificial dyeing, the processing of petroleum products; they enter the body along with the inhaled vapor, herbicides, asbestos dust, smog, ultraviolet radiation, x rays, and chemotherapy, Smoking, emotional stress, high physical activity and trauma, some medications, as well as in several other cases.
Normally, the body finds its own way of neutralizing free radicals. The misfortune occurs when free radicals it accumulates too many and the body will not be able to neutralize them.
The body defends itself
When in live tissue oxidizes, the body responds with the production of substances that surround the oxidant and monitor their destruction. These substances are called antioxidants. But if they exist, you may ask, why are people still sick? Why the violation has not always prevented by the vigilance of these internal defenders – the antioxidants?
Let's imagine a preschool where 30 three and four year old children only one teacher! It is in place in the allotted time. However, once children start to move actively, as the poor lady immediately gets crushed by the superior strength and energy. She constantly needed the next senior staff assistants. The same situation exists in the body against oxidation.
"Fighters" against the destructive action of free radicals are combined in the system antioxidant protection.
Ninety three million three hundred eighty four thousand seven hundred ninety
She's fighting for us on four levels:
first of all inhibits the formation of oxidants: oxygen is only sent to those areas where it is beneficial, and not ignored in an area where it can misbehave; it also stops the initiation of oxidation of metals, like iron (in the formation of free radicals are also included copper, cadmium, manganese, lead);
secondly, the security system intercepts the oxidant initiating the formation of Oh radicals and interrupts the chain reaction of reproduction numerous other oxidant;
thirdly eliminates the disruption caused by oxidants, which failed to intercept;
fourthly that eliminates and replaces the destroyed molecules, as well as self-cleaning, removing undesirable substances secreted in the course of their life.
The term "protection system of antioxidants" implies a close interdependence of the team effort defenders. The composition of players of the team include: bacteria, enzymes and nutrients.
1.Bacteria. Intestinal bacteria themselves cannot be considered as antioxidants. But they are decomposed by the biochemical substances, which can turn into oxidants. Thus, bacteria are our first protective strip.
2.Enzymes and nutrients. At the time of formation of the oxidant, a second protective strip. It is composed of enzymes — enzymes that destroy some of the most dangerous oxidants before they begin a chain reaction.
Seventy one million two hundred thirty nine thousand eight hundred seventy
Enzymes like explains nutritionist E. Somer, "similar to the equipment of the production line of the car; they speed up the Assembly process, without becoming part of the machine". Although the two substances with a sufficient amount of time can obviously interfere with each other and react, enzymes give confidence that this will happen and happen quickly. For example, "a chemical reaction, which can take hours or years to be accidental, in the presence of the enzyme would occur in many (thousands of times!) more quickly.» Our body produces millions of enzymes, and each of these enzymes is responsible only for one chemical reaction. However, it can carry out this reaction is not alone. Many enzymes have helpers called coenzymes or cofactors. Many cofactors are nutrients. Antioxidants cofactors include selenium, copper, Riboflavin, glutathione, coenzyme Q10, cysteine, manganese, zinc and bioflavonoids. All these nutrients can be found in a diet rich fruits, vegetables and whole grains. They help antioxidants-enzymes that protect our health.
Enzymes serve as a second band in our system for the protection of antioxidants, keeping the existing oxidants in low enough concentrations so that when carrying out their work they are unable to turn it into a wild, uncontrolled and destructive chain reaction.published
Author: Olena Eschenko
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: www.childneurologyinfo.com/health-text-biochem5.php
As the oxygen is doing it "dirty work", we say that an Apple and the car is oxidized.
Seventy four million six hundred ninety eight thousand nine hundred fourteen
The molecules that perform the function of oxidation, was called oxidants. Let's imagine that you left on the kitchen a warm pack of butter for another week. When you start to use it, it is probably the smell or the taste that the oil provarlo. This is another case of oxidant. The medical term for the oil or fat is a lipid. Scientists call the process of turning fresh fat rancid "oxidation of lipids in lipid peroxidation connection". Unfortunately, every cell of the body contains fat, which is gerontologist Dr. R. Welford calls "a private body butter" which at some time becomes rancid. The result can be "benign" — just faded skin, bad when it is lethal – cancer. The degree of destruction of an organism depends on many factors, including duration of response and the place where the act of "enemy". It also depends on genetic predisposition, the type of cellular nutrition, health and level of emotional stress of the individual. When a violation occurs in the blood vessels, it can cause disease of the capillaries. When it affects the DNA in the genetic information contained inside each cell nucleus, there may be defects or cancer. When the damaged lipids inside the lens of the eye, developed cataracts.
Thus, the oxygen earned the title of "universal oxidant" and I think the list of diseases and conditions caused by his action, becomes every day longer.
Charged free radicals
Oxidants are often free radicals. Free from what? Chemists are called radicals, tiny particles that maintain their uniqueness. In the body free radical "free" because it loses an electron.
Maybe you have seen the picture of the atom with something orbiting the nucleus? This something is an electron.
Molecules are stable when they have equal pairs of electrons (paired electrons). When a molecule loses an electron it becomes a free radical and hard and unintelligible "steals" the missing electron. Unfortunately, theft victim also becomes a free radical and immediately begins the search for his electron to the next theft, creating another free radical.
Eight million eight hundred six thousand two hundred twelve
Formed a cascade of electronic theft, and it leads to the destruction of the tissues of the body. In addition, this destructive chain reaction creates new compounds which also make a mess. Since pickpocket is the best place in the crowd, free radicals also prefer a region where accumulates a large number of electrons.
Free radicals are especially partial to polyunsaturated fatty acids, which make up about half the fat content of the membrane surrounding every cell in your body.
Where do free radicals
Free radicals are born in the process of cellular metabolism, which is in regular daily elimination of various defects, the intake of nutrients, energy production, reproduction, disposal of waste remaining after the implementation of all other functions.
Free radicals are formed also in the use of alcohol, cured meats; they appear during the artificial dyeing, the processing of petroleum products; they enter the body along with the inhaled vapor, herbicides, asbestos dust, smog, ultraviolet radiation, x rays, and chemotherapy, Smoking, emotional stress, high physical activity and trauma, some medications, as well as in several other cases.
Normally, the body finds its own way of neutralizing free radicals. The misfortune occurs when free radicals it accumulates too many and the body will not be able to neutralize them.
The body defends itself
When in live tissue oxidizes, the body responds with the production of substances that surround the oxidant and monitor their destruction. These substances are called antioxidants. But if they exist, you may ask, why are people still sick? Why the violation has not always prevented by the vigilance of these internal defenders – the antioxidants?
Let's imagine a preschool where 30 three and four year old children only one teacher! It is in place in the allotted time. However, once children start to move actively, as the poor lady immediately gets crushed by the superior strength and energy. She constantly needed the next senior staff assistants. The same situation exists in the body against oxidation.
"Fighters" against the destructive action of free radicals are combined in the system antioxidant protection.
Ninety three million three hundred eighty four thousand seven hundred ninety
She's fighting for us on four levels:
first of all inhibits the formation of oxidants: oxygen is only sent to those areas where it is beneficial, and not ignored in an area where it can misbehave; it also stops the initiation of oxidation of metals, like iron (in the formation of free radicals are also included copper, cadmium, manganese, lead);
secondly, the security system intercepts the oxidant initiating the formation of Oh radicals and interrupts the chain reaction of reproduction numerous other oxidant;
thirdly eliminates the disruption caused by oxidants, which failed to intercept;
fourthly that eliminates and replaces the destroyed molecules, as well as self-cleaning, removing undesirable substances secreted in the course of their life.
The term "protection system of antioxidants" implies a close interdependence of the team effort defenders. The composition of players of the team include: bacteria, enzymes and nutrients.
1.Bacteria. Intestinal bacteria themselves cannot be considered as antioxidants. But they are decomposed by the biochemical substances, which can turn into oxidants. Thus, bacteria are our first protective strip.
2.Enzymes and nutrients. At the time of formation of the oxidant, a second protective strip. It is composed of enzymes — enzymes that destroy some of the most dangerous oxidants before they begin a chain reaction.
Seventy one million two hundred thirty nine thousand eight hundred seventy
Enzymes like explains nutritionist E. Somer, "similar to the equipment of the production line of the car; they speed up the Assembly process, without becoming part of the machine". Although the two substances with a sufficient amount of time can obviously interfere with each other and react, enzymes give confidence that this will happen and happen quickly. For example, "a chemical reaction, which can take hours or years to be accidental, in the presence of the enzyme would occur in many (thousands of times!) more quickly.» Our body produces millions of enzymes, and each of these enzymes is responsible only for one chemical reaction. However, it can carry out this reaction is not alone. Many enzymes have helpers called coenzymes or cofactors. Many cofactors are nutrients. Antioxidants cofactors include selenium, copper, Riboflavin, glutathione, coenzyme Q10, cysteine, manganese, zinc and bioflavonoids. All these nutrients can be found in a diet rich fruits, vegetables and whole grains. They help antioxidants-enzymes that protect our health.
Enzymes serve as a second band in our system for the protection of antioxidants, keeping the existing oxidants in low enough concentrations so that when carrying out their work they are unable to turn it into a wild, uncontrolled and destructive chain reaction.published
Author: Olena Eschenko
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: www.childneurologyinfo.com/health-text-biochem5.php
5 simple exercises will relieve pain and numbness in the cervical spine
From Kamchatka to Africa for a second. When the photographer loves the North and the South