526
Coconut will help to store hydrogen efficiently and safely

Hydrogen is clean fuel. Its implementation, among other issues, hindered by the lack of effective storage technology. According to a recent study, coconut contains "secret ingredients" that will help to overcome difficulties.
Inventory of hydrogen on the planet is practically inexhaustible, because it is part of the most common on Earth of the substance of water. In addition, by burning hydrogen we get the same water, and do not cause any damage to the environment. Due to these properties, hydrogen has a chance to displace fuel derived from fossil hydrocarbons.
But on the way to that bright dream of a lot of difficulties. One of them — have not yet learned to effectively, reliably and safely store hydrogen has a low volumetric energy density. For example, in one liter of petroleum gasoline, hydrogen, loosely speaking, contains 60% more than one liter of pure liquid hydrogen. In other words, hydrogen will always require larger tanks than gasoline.
Hydrogen storage is a huge problem. To increase the density of it could be liquefied, but hydrogen boils at a temperature of -250oC. To keep it in a liquid state requires a strong and bulky insulation.
Compressed hydrogen is dangerous, therefore, this technology is unsuitable for widespread adoption in transportation, where major and minor accidents and traffic accidents occur very often.
Due to the fact that liquefaction and compression of hydrogen is not able to solve the problem, science has been developing technologies for chemical storage. Scientists are looking for materials that can effectively adsorb hydrogen and release it as needed.
At first everyone's attention was attracted by the metal hydrides. But it turned out that they have flaws. To release hydrogen, these materials must be heated, and hence inefficient use of energy. In addition, metal hydrides can be recharged a limited number of times, about 100, after which they quickly lose capacity with increase in the number of recharges.
Vini Dixit (Viney Dixit) and his colleagues from the Centre for hydrogen energy at the University in Varanasi, India, reported about the opening. They found that charcoal made from coconut pulp can perfectly cope with the problem of hydrogen storage. At least, the ability to adsorb hydrogen, this material is not worse than others, but "coconut coal" keeps working after multiple recharge cycles.
Carbon readily binds to the hydrogen, and just as easily releases it when it is necessary. In addition, carbon is easy to make a porous material with high surface area.
One of the ways of obtaining coal with the desired properties is the "carbonization" of biological material, e.g. of pulp fruit or coconut shell. The method consists in heating the raw material to several hundred degrees Celsius in a nitrogen atmosphere, guaranteeing the preservation of the carbon and its porous biological structure.
Instead of the coconut shell Dixie used his flesh. Its advantage is the wide range of additional elements such as potassium, magnesium, sodium and calcium, which are evenly distributed throughout the volume of the material. According to the researchers, this feature contributes to the fact that carbon binds more hydrogen.
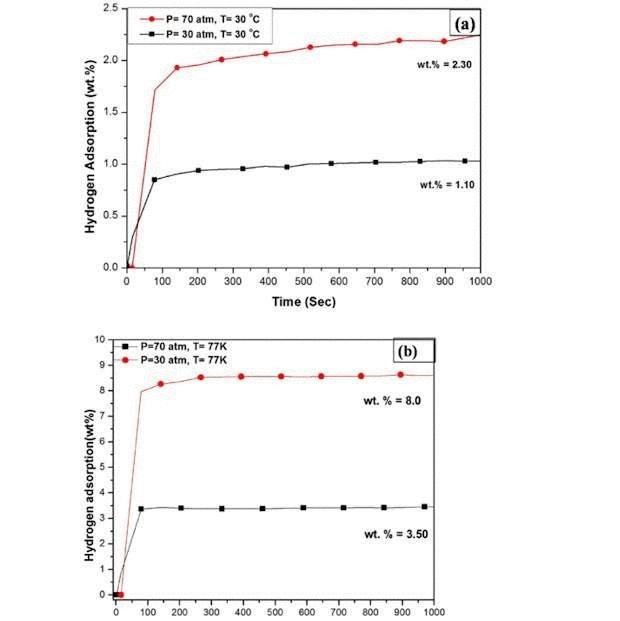
As reported by scientists, their "coconut" carbon adsorbs 2,3% hydrogen by weight at room temperature and 8% at the temperature of liquid nitrogen under a pressure of 70 atmospheres.
According to the criteria developed by the us Department of energy, a viable technology is the one with which can be created a system capable of storing not less than 5.5% hydrogen.
It is obvious that Indian scientists have not yet reached the targets, because the result of their work is not a practical system, and only material that may be her base. However, they were able to "find" an important new movement and direction for further research to find the dependence of the adsorption property of the carbon catalysts.
Source: facepla.net