575
The new catalyst allows you to use hydrogen as the storage of solar and wind energy
Most renewable energy sources has certain disadvantages - they are sometimes too dependent on weather conditions and time of day, that is, their consistency is poor. Very convenient to get energy from the sun, but if cloudy outside? You can use wind energy, but what to do when a lull?
If it was possible to store surplus energy generated during a particularly sunny or windy days, it was possible to use these resources whenever you need it - erasing the advantage of such "traditional" sources, which supply energy to the extent necessary, as nuclear energy and others.
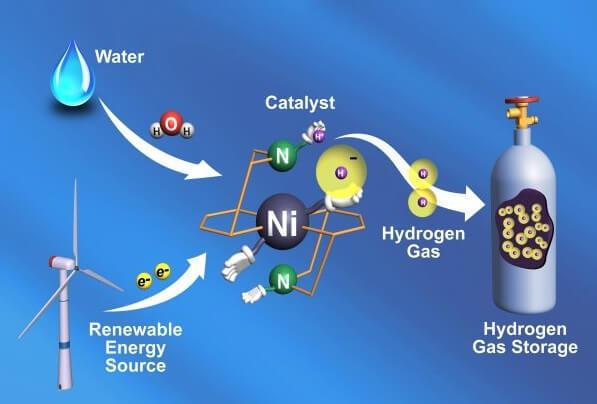
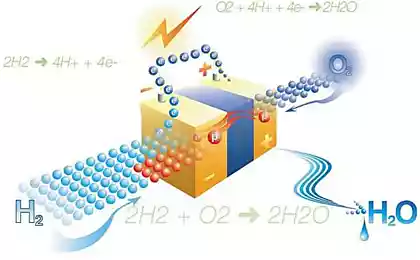
But there's one original way to solve this problem - to use electricity generated due to solar or wind action, for the flow of the electrolytic reaction, in fact, for the decomposition of water into oxygen and hydrogen; hydrogen can then be isolated and accumulate as a backup fuel source.
Recently, a team of scientists from SLAC National Laboratory and the University of Toronto, an important step to make the process easier and more efficient. With the help of powerful computers they created an electrolytic catalyst which is three times more efficient than previous models.
Metallic gel
The new technology is based on increasing the efficiency of the catalyst zheleznokobaltovogo due to the simple addition of tungsten. It sounds easy enough in theory, but much more difficult in practice. Computer simulations showed that the catalyst must be thoroughly mixed, these three elements in order to ensure maximum activity on the surface of the reaction.
Researchers obtained by dissolving a mixture of three metals in the solution, which was then allowed to stand at room temperature until a gel state, thus preventing the formation of separate clusters of metal atoms. Finally, the gel was dried and the powder made therefrom having increased porosity, thus increasing the surface area available for catalytic reactions. A new catalyst produces the oxygen is three times faster than previous versions, and, importantly, it can do so for hundreds of reactions.
"This significant progress, although there is still much room for improvement, - said Professor of Electrical and Computer Engineering University of Toronto Edward Sargent. - We need to make catalysts and electrolysis system even more effective, efficient and productive in order to reduce the cost of production of renewable hydrogen fuel to
competitive level. " Nevertheless, this is a big step forward in the field of energy for ensuring environmental well-being in the future.
If it was possible to store surplus energy generated during a particularly sunny or windy days, it was possible to use these resources whenever you need it - erasing the advantage of such "traditional" sources, which supply energy to the extent necessary, as nuclear energy and others.

But there's one original way to solve this problem - to use electricity generated due to solar or wind action, for the flow of the electrolytic reaction, in fact, for the decomposition of water into oxygen and hydrogen; hydrogen can then be isolated and accumulate as a backup fuel source.
Recently, a team of scientists from SLAC National Laboratory and the University of Toronto, an important step to make the process easier and more efficient. With the help of powerful computers they created an electrolytic catalyst which is three times more efficient than previous models.
Metallic gel
The new technology is based on increasing the efficiency of the catalyst zheleznokobaltovogo due to the simple addition of tungsten. It sounds easy enough in theory, but much more difficult in practice. Computer simulations showed that the catalyst must be thoroughly mixed, these three elements in order to ensure maximum activity on the surface of the reaction.
Researchers obtained by dissolving a mixture of three metals in the solution, which was then allowed to stand at room temperature until a gel state, thus preventing the formation of separate clusters of metal atoms. Finally, the gel was dried and the powder made therefrom having increased porosity, thus increasing the surface area available for catalytic reactions. A new catalyst produces the oxygen is three times faster than previous versions, and, importantly, it can do so for hundreds of reactions.
"This significant progress, although there is still much room for improvement, - said Professor of Electrical and Computer Engineering University of Toronto Edward Sargent. - We need to make catalysts and electrolysis system even more effective, efficient and productive in order to reduce the cost of production of renewable hydrogen fuel to
competitive level. " Nevertheless, this is a big step forward in the field of energy for ensuring environmental well-being in the future.