820
Stanford improved cheapest way water electrolysis

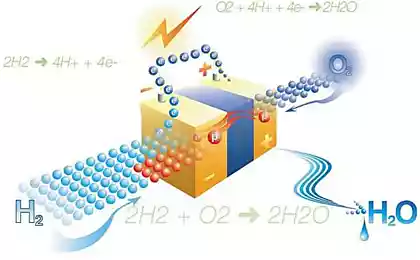
Last August, scientists from Stanford University have demonstrated for the first time inexpensive way of electrolysis of water, that is, the separation of H 2 sub> O into hydrogen and oxygen. To initiate a chemical process rather simple AAA batteries. Of course, batteries may be used instead of a small solar panel which provides a potential difference at least 1, 5 volts.
In the past, scientists have used the cathodes and anodes of nickel and nickel oxide. This is the first in the world experience when electrolysis was able to abandon the electrodes made of precious metals (platinum, iridium), and when the process was going at such a low voltage.
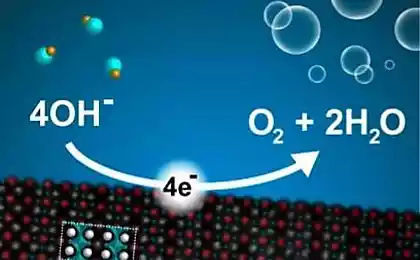
Now they were able to reduce the cost and simplify more electrolysis, which would make hydrogen fuel more cheaply if the display technology on an industrial scale. In the improved process technology for cathode and anode used the same catalyst from NiFeO x sub>. That is, the anode and cathode is no longer require different pH-factor (one acidic, alkaline other) so that they are easily and conveniently be placed in a common vessel with water. We can only collect produce oxygen and hydrogen (although it is better not to collect oxygen, and immediately to release to the atmosphere).
The video below shows how the electrolysis of AAA batteries. With one electrode, oxygen, and with another - hydrogen. Authors of scientific work say that the intensity of the reaction is even higher than with the combination of the traditional electrode iridium oxide and platinum.
Reaction goes steadily and very active on the entire surface of the electrodes.
The secret of "focus" - in the structure of the catalyst NiFeO x sub>. While this seemingly simple materials, but the structure of the material is very specific. He somehow "grown" on carbon nanofibres (of research it is not clear how to make it). Scientists say that this wonderful catalyst in the future can be adapted for other chemical reactions, except for water electrolysis.
Although this technology looks very unlikely, as all eight co-authors of scientific work - the Chinese, we should not forget that they are working in the Department of Materials Science and Technology, Stanford University, one of the most prestigious scientific institutions in the world.
The catalytic reaction of the metal oxide nanoparticles (iron, cobalt, nickel oxides or mixtures of their oxides) of about 20 nm is electrochemically converted into ultrasmall nanoparticles NiFeO x sub> with a diameter of 2-5 nm as a result of reactions induced by lithium. Unlike traditional chemical synthesis, the authors of the invention, this method allows to maintain a superior electrical connection between the nanoparticles and results in the formation of large area for the catalytic reaction.
During the experiment was tested continuous operation of the device in this way for a week (200 hours) without degradation of the electrodes, говорит And Tsui (Yi Cui), one of the authors of scientific work. He added that the efficiency of the electrolysis of water is 82% at room temperature (apparently, under normal pressure, too).
Results of the study опубликованы June 23, 2015 in the journal Nature Communications (in free access).
As noted a year ago, this is a very important project because it greatly simplifies the manufacturing technology of fuel cells and hydrogen. In such cells can operate mobile electronics, and automobiles.

Toyota Mirai, one of the world's first car on hydrogen fuel cells. Sales began 15.12.2014, under the bottom of his two cylinders with hydrogen at a pressure of 70 MPa. Refueling takes 3-5 minutes. Range of speed: 480 km i>
It is important that the combustion of hydrogen the only by-product of combustion is water. The same water that is split into components, such as sunlight on the first stage of the technical process.
Source: geektimes.ru/post/252516/
Fallout fans will get the game from Bethesda Fallout 4 for their caps from bottles
Investment Trends: Robotics boom