475
The splitting of water with 100% efficiency: half done
If you find a cheap and easy method of electrolysis/photolysis of water, we get incredibly rich and clean energy source — hydrogen fuel. Burning in oxygen, hydrogen does not form any side emissions, in addition to water. Theoretically, electrolysis is a very simple process: it is enough to pass the electric current through the water, and it is split into hydrogen and oxygen. But now, all the developed technological process require such a large amount of energy that electrolysis becomes unprofitable.

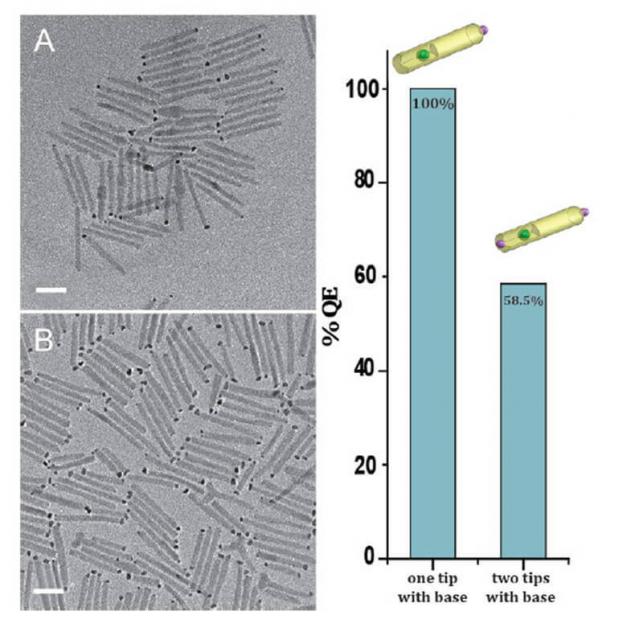
Now scientists have solved part of the puzzle. Researchers from the Technion-Israel Institute of technology have developed a method of carrying out the second two steps of the redox reaction, recovery in the visible (solar) light with an energy efficiency of 100%, significantly surpassing the previous record of 58.5%.
It remains to improve the oxidation half-reaction.
Such a high efficiency has been achieved due to the fact that the process uses only light energy. Catalysts (photocatalysts) are nanorods with a length of 50 nm. They absorb the photons from the light source and give electrons.
In the half-reaction of oxidation produced four separate hydrogen atoms and a molecule of O2 (which is not needed). In the half-reaction recovery of four hydrogen atoms paired in the two molecules H2, producing a useful form of hydrogen gas H2,
The effectiveness of 100% means that all the photons received by the system are involved in the generation of electrons.
At this efficiency, each Anasterian generates about 100 molecules of H2 per second.
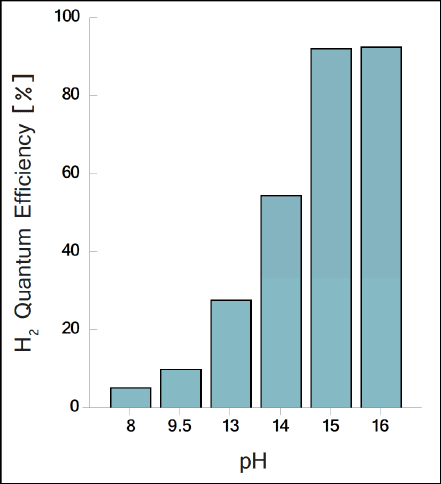
Now scientists are working on optimization of process technology, which so far requires an alkaline environment with an incredibly high pH. This level not acceptable for real-world conditions.
In addition, the nanorods subject to corrosion, which is also not too good.
However, today humanity is one step closer to getting inexhaustible source of clean energy in the form of hydrogen fuel. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/271810/

Now scientists have solved part of the puzzle. Researchers from the Technion-Israel Institute of technology have developed a method of carrying out the second two steps of the redox reaction, recovery in the visible (solar) light with an energy efficiency of 100%, significantly surpassing the previous record of 58.5%.
It remains to improve the oxidation half-reaction.
Such a high efficiency has been achieved due to the fact that the process uses only light energy. Catalysts (photocatalysts) are nanorods with a length of 50 nm. They absorb the photons from the light source and give electrons.
In the half-reaction of oxidation produced four separate hydrogen atoms and a molecule of O2 (which is not needed). In the half-reaction recovery of four hydrogen atoms paired in the two molecules H2, producing a useful form of hydrogen gas H2,
The effectiveness of 100% means that all the photons received by the system are involved in the generation of electrons.
At this efficiency, each Anasterian generates about 100 molecules of H2 per second.

Now scientists are working on optimization of process technology, which so far requires an alkaline environment with an incredibly high pH. This level not acceptable for real-world conditions.

In addition, the nanorods subject to corrosion, which is also not too good.
However, today humanity is one step closer to getting inexhaustible source of clean energy in the form of hydrogen fuel. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/271810/
The myth of women's intuition
Scientists of MIT have developed ultralight solar panels outstanding to 6 watts per gram of weight