1097
Invented cheapest way water electrolysis
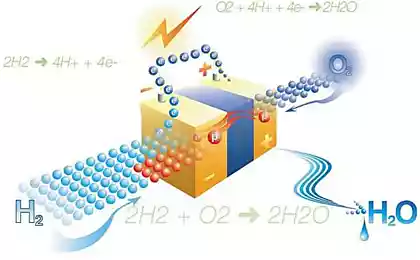
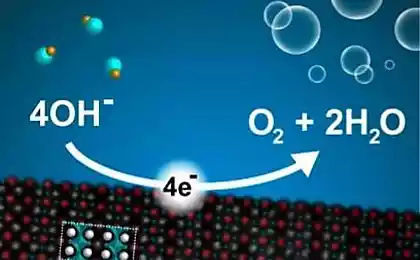
Scientists from Stanford University have demonstrated an inexpensive way of electrolysis of water, ie the separation of H 2 sub> O for oxygen and hydrogen. To initiate a chemical process rather simple AAA batteries.
Scientists use cathodes and anodes of nickel and nickel oxide, thus significantly reducing the supply voltage.
Stanford experience - the first in the world when the electrolysis was able to abandon the electrodes made of precious metals (platinum, iridium), and when the process goes at such a low voltage.
Results of the study опубликованы in the journal Nature Communications (DOI: 10.1038 / ncomms5695).
This invention is very important because it simplifies the process of manufacturing the fuel cell with hydrogen. In one such cell, for example, a mobile phone can work for several decades. If we take for example a car, it will never need to refuel (perhaps once every two or three years of intensive use). Significantly, using hydrogen fuel cells only byproduct of combustion is water.
Source: habrahabr.ru/post/234523/
Scientists use cathodes and anodes of nickel and nickel oxide, thus significantly reducing the supply voltage.
Stanford experience - the first in the world when the electrolysis was able to abandon the electrodes made of precious metals (platinum, iridium), and when the process goes at such a low voltage.
Results of the study опубликованы in the journal Nature Communications (DOI: 10.1038 / ncomms5695).
This invention is very important because it simplifies the process of manufacturing the fuel cell with hydrogen. In one such cell, for example, a mobile phone can work for several decades. If we take for example a car, it will never need to refuel (perhaps once every two or three years of intensive use). Significantly, using hydrogen fuel cells only byproduct of combustion is water.
Source: habrahabr.ru/post/234523/