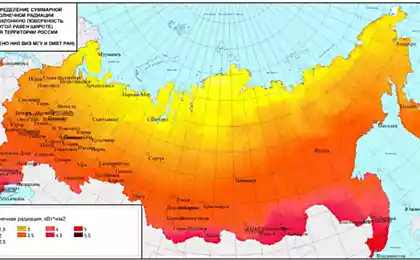
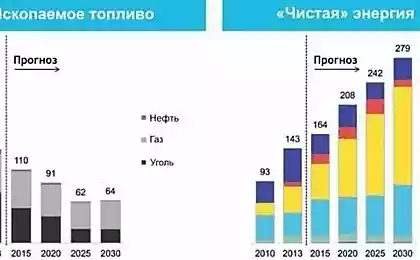
476
Molecular approach to solar energy
Scientists from mit and Harvard University have developed a way of storing solar energy in molecules that can be used for heating houses or cooking. Molecules are always able to keep warm and can be used unlimited number of times without producing any greenhouse gas emissions. While we are not talking about commercial use of the method, but the researchers were able to demonstrate under laboratory conditions the performance of the phenomenon called foroperational (photoswitching).
"Some molecules, known as fotoprikoly can take either of two different shapes, as if they had a hinge in the middle — written by MIT researchers in an article published in the journal Nature Chemistry. — Exposure to sunlight leads to energy absorption and transition from one configuration to the other, which remains stable for long periods of time."
All that is necessary for the release of energy, is to expose the molecules to the impact of a small amount of heat, electricity or light, after which they return to their original state while emitting heat. "In fact, they behave as rechargeable thermal batteries, taking solar energy, keeping it indefinitely, and releasing on demand", — said in the article.

The researchers used photopracticum a substance called azobenzene, attaching molecules on a substrate of carbon nanotubes. The task was to place the molecules so close to each other, to achieve energy densities sufficient for generating useful heat. But in practice, has managed to package together only half of the required number of molecules, calculated using computer simulation. Surprisingly, instead of the projected growth of the energy density by 30% during the experiment, it increased by 200%.
It turned out that the important thing is not how many molecules of azobenzene fit on a single carbon nanotube, but how nanotubes are close to each other. The azobenzene forms on the surface "teeth" that blocks adjacent nanotubes together. The result is the concentration needed for usable energy. According to the researchers, changing the combination fotoprikoly molecules and the substrate it is possible to obtain greater or lesser amount of energy. What benefits can bring the use of fotoprikoly in practice? The study's lead author, doctoral student, MIT and Harvard Timothy Kucharski (Timothy Kucharski) believes that, most likely, it will be possible to store energy in a liquid form, convenient for transportation.
"It will be possible to charge the liquid material in the tank through a window or transparent channel from the sun, and then move to another storage tank, where the material will remain as long as necessary, says Kucharski. Thus, it would be possible to accumulate the charged material for use in a time when the sun does not Shine." According to the authors of the study, the technology could replace the burning of firewood for cooking, creating dangerous concentrations of pollutants within the areas, leading to deforestation and contributing to climate change.
Source: facepla.net
"Some molecules, known as fotoprikoly can take either of two different shapes, as if they had a hinge in the middle — written by MIT researchers in an article published in the journal Nature Chemistry. — Exposure to sunlight leads to energy absorption and transition from one configuration to the other, which remains stable for long periods of time."
All that is necessary for the release of energy, is to expose the molecules to the impact of a small amount of heat, electricity or light, after which they return to their original state while emitting heat. "In fact, they behave as rechargeable thermal batteries, taking solar energy, keeping it indefinitely, and releasing on demand", — said in the article.

The researchers used photopracticum a substance called azobenzene, attaching molecules on a substrate of carbon nanotubes. The task was to place the molecules so close to each other, to achieve energy densities sufficient for generating useful heat. But in practice, has managed to package together only half of the required number of molecules, calculated using computer simulation. Surprisingly, instead of the projected growth of the energy density by 30% during the experiment, it increased by 200%.
It turned out that the important thing is not how many molecules of azobenzene fit on a single carbon nanotube, but how nanotubes are close to each other. The azobenzene forms on the surface "teeth" that blocks adjacent nanotubes together. The result is the concentration needed for usable energy. According to the researchers, changing the combination fotoprikoly molecules and the substrate it is possible to obtain greater or lesser amount of energy. What benefits can bring the use of fotoprikoly in practice? The study's lead author, doctoral student, MIT and Harvard Timothy Kucharski (Timothy Kucharski) believes that, most likely, it will be possible to store energy in a liquid form, convenient for transportation.
"It will be possible to charge the liquid material in the tank through a window or transparent channel from the sun, and then move to another storage tank, where the material will remain as long as necessary, says Kucharski. Thus, it would be possible to accumulate the charged material for use in a time when the sun does not Shine." According to the authors of the study, the technology could replace the burning of firewood for cooking, creating dangerous concentrations of pollutants within the areas, leading to deforestation and contributing to climate change.
Source: facepla.net