450
Toyota claims that magnesium could replace lithium
Engineers at the research Institute Toyota North America (Trina) believe they have found the secret use of magnesium in batteries. This will allow to replace lithium safer, more energy-intensive option in all batteries, from mobile phones to cars.
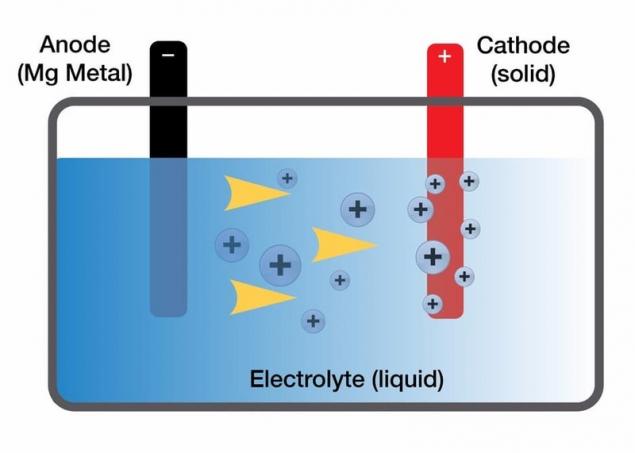
Magnesium was considered as a potential option to replace lithium in batteries for some time. Lithium is not stable in air and can be ignited when it is exposed, therefore, to make lithium ion batteries safer, the number of lithium ions is reduced and integrates directly into the graphite rods. This reduces the amount of metal (reducing the density), which limits the amount of energy that lithium-ion battery can store.
To increase the density of the engineers experimented with a compound of lithium and graphite, a form element, but to achieve a balance was extremely difficult.

Magnesium, on the other hand, is stable in the atmosphere and potentially more energy intensive than lithium, from the point of view of energy storage. The trouble is that, to form the electrolyte in which magnesium is not degraded, and which will provide efficient transmission of energy was a difficult task. But the situation changed after the accidental discovery during research in the field of hydrogen fuel cells.

Chief scientist and chemical engineer Toyota Mohtadi Rana (Rana Mohtadi) overheard discussion colleagues on the problem of development-friendly magnesium electrolyte. She realized that material properties for hydrogen storage, which she, perhaps, could be applied to the battery based on magnesium. She gathered a team and began work to test this theory.
Head of research group-Toyota, Paul Fanson (Paul Fanson), assigns the discovery to several researchers of the Institute and team work.

The team prepared a paper describing the discovery, which was published in the journal Angewandte Chemie International Edition. They hope that other researchers outside Toyota will be able to make use of the material and to accelerate the development of batteries based on magnesium for everyday use.
"Unlocking the full potential of rechargeable magnesium batteries was partially hampered by the dependence of complex systems on the basis of the chlorides. Despite the high anodic stability of these electrolytes, they cause corrosion of metal components of the battery, which reduce the properties of their electrochemical "window". Following our new design concept, with the participation of cluster anions of boron, monocarbon CB11H12 (-) produces halogen-free, simple type of magnesium salt which is compatible with metallic magnesium and shows oxidation stability superior to the ether solvents. Due to its inertness and non-aggressive nature of the electrolyte on the basis of magnesium helps to standardize methods of high voltage testing of the cathode, which uses a typical flat round battery. This achievement is a turning point in the research and development of magnesium electrolytes, which will have a direct impact on the physical implementation of rechargeable magnesium batteries".
While it is necessary to follow a certain path, before we install the batteries based on magnesium in our smartphones, what do researchers have to wait about 20 years before these batteries will become mainstream. It is hoped that the presentation to the public of such discoveries can help to speed up the process.
SUBSCRIBE to OUR youtube channel that allows you to watch online, download from YouTube free video about the recovery, the rejuvenation of man. Love for others and ourselves, as the feeling of high vibrations — an important factor for improvement .
http://cdn00.vidyomani.com/c/4/7/8/yaywpcvvuf9k/index.html
Put LIKES and share with your FRIENDS!
www.youtube.com/channel/UCXd71u0w04qcwk32c8kY2BA/videos
Subscribe -https://www.facebook.com//
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: facepla.net/the-news/tech-news-mnu/5468-toyota-%D0%BC%D0%B0%D0%B3%D0%BD%D0%B8%D0%B9.html

Magnesium was considered as a potential option to replace lithium in batteries for some time. Lithium is not stable in air and can be ignited when it is exposed, therefore, to make lithium ion batteries safer, the number of lithium ions is reduced and integrates directly into the graphite rods. This reduces the amount of metal (reducing the density), which limits the amount of energy that lithium-ion battery can store.
To increase the density of the engineers experimented with a compound of lithium and graphite, a form element, but to achieve a balance was extremely difficult.

Magnesium, on the other hand, is stable in the atmosphere and potentially more energy intensive than lithium, from the point of view of energy storage. The trouble is that, to form the electrolyte in which magnesium is not degraded, and which will provide efficient transmission of energy was a difficult task. But the situation changed after the accidental discovery during research in the field of hydrogen fuel cells.

Chief scientist and chemical engineer Toyota Mohtadi Rana (Rana Mohtadi) overheard discussion colleagues on the problem of development-friendly magnesium electrolyte. She realized that material properties for hydrogen storage, which she, perhaps, could be applied to the battery based on magnesium. She gathered a team and began work to test this theory.
Head of research group-Toyota, Paul Fanson (Paul Fanson), assigns the discovery to several researchers of the Institute and team work.

The team prepared a paper describing the discovery, which was published in the journal Angewandte Chemie International Edition. They hope that other researchers outside Toyota will be able to make use of the material and to accelerate the development of batteries based on magnesium for everyday use.
"Unlocking the full potential of rechargeable magnesium batteries was partially hampered by the dependence of complex systems on the basis of the chlorides. Despite the high anodic stability of these electrolytes, they cause corrosion of metal components of the battery, which reduce the properties of their electrochemical "window". Following our new design concept, with the participation of cluster anions of boron, monocarbon CB11H12 (-) produces halogen-free, simple type of magnesium salt which is compatible with metallic magnesium and shows oxidation stability superior to the ether solvents. Due to its inertness and non-aggressive nature of the electrolyte on the basis of magnesium helps to standardize methods of high voltage testing of the cathode, which uses a typical flat round battery. This achievement is a turning point in the research and development of magnesium electrolytes, which will have a direct impact on the physical implementation of rechargeable magnesium batteries".
While it is necessary to follow a certain path, before we install the batteries based on magnesium in our smartphones, what do researchers have to wait about 20 years before these batteries will become mainstream. It is hoped that the presentation to the public of such discoveries can help to speed up the process.
SUBSCRIBE to OUR youtube channel that allows you to watch online, download from YouTube free video about the recovery, the rejuvenation of man. Love for others and ourselves, as the feeling of high vibrations — an important factor for improvement .
http://cdn00.vidyomani.com/c/4/7/8/yaywpcvvuf9k/index.html
Put LIKES and share with your FRIENDS!
www.youtube.com/channel/UCXd71u0w04qcwk32c8kY2BA/videos
Subscribe -https://www.facebook.com//
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: facepla.net/the-news/tech-news-mnu/5468-toyota-%D0%BC%D0%B0%D0%B3%D0%BD%D0%B8%D0%B9.html