411
Amazing transformations of nitrates in the body
Nitrates are strongly associated with evil, machinations, diseases and we see at any occasion. There are quite extensive studies that indicate a connection between the amount of nitrates and the development of, for example, stomach cancer. But a meta-analysis of these studies still does not give grounds to assert with certainty that nitrates are one hundred percent carcinogenic. The optimal modern diet recommended by the World Health Organization contains about 500 grams of vegetables and fruits. So, scientists from the universities of Michigan and Texas have estimated that it is about 55% higher than the daily nitrate rate established by the same WHO. Paradox? Nope. It’s all about their number and context. Further changes in nitrates in the human body depend on a number of external conditions.“Everything is poison, and everything is medicine. The dose alone makes a substance either a poison or a medicine, is the statement of Paracelsus. We will understand nitrates, because of all the harmful substances in products, nitrates are known more than others, and many people have a real nitratophobia.Nitrates and other nitrogen compounds in nature.The nitrogen cycle is a series of closed interconnected pathways along which nitrogen circulates in the Earth’s biosphere. Let us first consider the process of decomposition of organic substances in the soil. Various microorganisms extract nitrogen from decaying materials and convert it into molecules they need for metabolism. The remaining nitrogen is released as ammonia (NH3) or ammonium ions (NH4+). Other microorganisms then bind this nitrogen, converting it to the form of nitrates (NO3-). Entering plants (and eventually entering the organisms of living things), this nitrogen is involved in the formation of biological molecules.
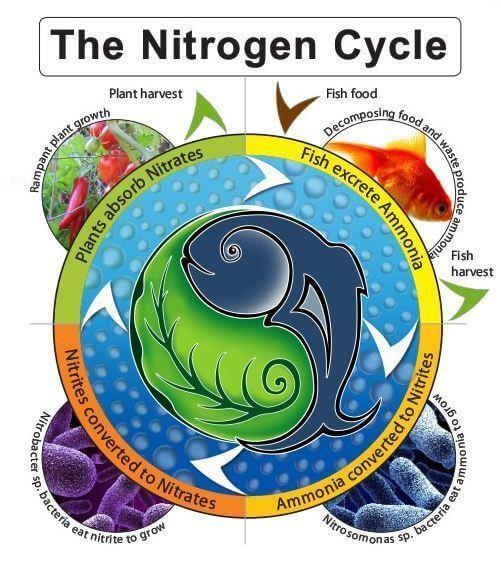
After the body dies, nitrogen returns to the soil, and the cycle begins again. During this cycle, both nitrogen losses — when it is incorporated into sediments or released during the life of certain bacteria (called denitrifying bacteria) — and compensation for these losses through volcanic eruptions and other geological activities are possible. But most of the bound nitrogen man produces in the form of mineral fertilizers. Nitrogen deficiency often inhibits plant growth, and farmers buy artificially bound nitrogen in the form of mineral fertilizers to increase yields. Now for agriculture every year produces a little more than 80 million tons of bound nitrogen (note that it is used not only for growing food crops – suburban lawns and gardens fertilize them). Summarizing the entire human contribution to the nitrogen cycle, we get a figure of about 140 million tons per year. Approximately the same amount of nitrogen binds naturally in nature. Thus, in a relatively short period of time, man began to exert a significant influence on the nitrogen cycle in nature. What are the consequences? Each ecosystem is able to absorb a certain amount of nitrogen, and the consequences are generally favorable - plants will grow faster. However, when the ecosystem is saturated, nitrogen will begin to wash out into rivers and accumulate in excess in plants. But more on that later.
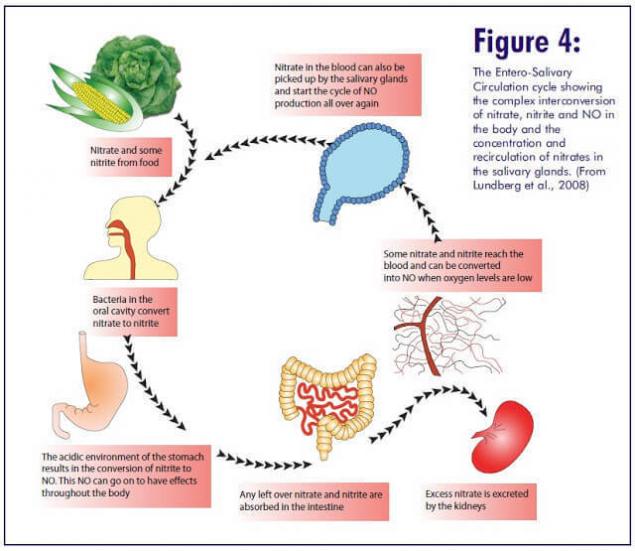
General characteristics of the nitrate pathway in the human body.Sources of nitrite intake in the stomach were studied on the example of the usual diet of a resident of the United States. The largest amount of dietary nitrates comes from vegetables - more than 85%. About 25% of food nitrates are involved in the cycle between the gut and saliva. Approximately 20% of nitrates under the influence of oral microflora are reduced to nitrites, which make up about 80% of all nitrites present in the contents of the stomach. Approximately 20% of nitrites are components of the daily diet. They found that the bulk of the nitrates contained in the eaten food products are absorbed in the digestive tract unchanged, enters the blood, and from it into the cells and tissues of the body. But within 6-8 hours the absorbed nitrates are almost completely excreted from the body by the kidneys. The remaining in the digestive tract, a smaller part of nitrates, indeed, with the help of microorganisms living in the digestive system, turns into nitrites. But these nitrites do not enter into undesirable reactions with the formation of carcinogenic substances - nitrosamines, as previously thought.
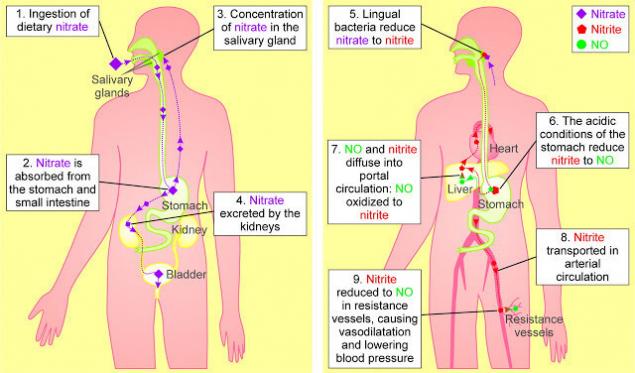
Nitric oxide (NO) is one of the strongest vasodilators and plays a key role in many significant processes – the regulation of vascular tone, pressure, platelet aggregation, mitochondrial respiration, nervous regulation and a number of others. NO synthesis is carried out in two main ways – related to the activity of the special enzyme NO synthase (NOS) and independent of the activity of NO synthase method. In addition to the intake of nitrates from food, they are also endogenously formed in the body during the oxidation of NO. The normal level of nitrates in the blood is 20-40 μM, and the level of nitrites is much lower - 50-1000 nM. The half-life of circulating nitrates in the blood is about 5 hours. It is still unclear how nitrates are actively absorbed by the salivary glands and their concentration in saliva is 20 times higher than in the blood. In the oral cavity, optional anaerobic bacteria restore nitrates to nitrites using the nitrate reductase enzyme.
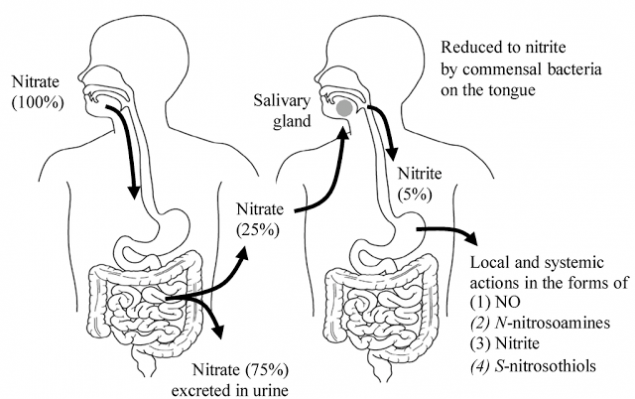
Oral cavity, nitrates and nitrites.A significant amount of nitrates that are absorbed in the intestine enters the blood and is excreted with saliva. At the same time, the process of isolation goes not only during meals, but also in other hours. The transformation of nitrates primarily occurs in the oral cavity. Among the bacteria that inhabit the oral cavity, there are species that are able to restore nitric acid salts first to nitrite, and then to ammonium cation, through the formation of a number of intermediate products. That is, in the oral cavity there are microorganisms that produce enzymes nitratereductase and nitritreductase. The main producer of nitreductase in the oral cavity are bacteria of the genus Veillonella spp. Representatives of this kind of anaerobic bacteria dispose of lactic and other organic acids formed by streptococci, lactobacilli, yeast and actinomycetes, counteract the displacement of pH in the acid side due to lactic acid fermentation. That is, the detection in the oral cavity of Waylonella is a prognostically good sign. It is possible that with the risk of caries, the activity of nitreductase decreases. Studying the fate of nitrates in the human body, British biochemists have found that up to a quarter of all nitrates eaten with blood return to the cells of the oral cavity and are released into saliva. Here they turn into nitrites and saliva enter the digestive tract. Approximately 20% of nitrates under the influence of oral microflora are reduced to nitrites, which make up about 80% of all nitrites present in the contents of the stomach. Surprisingly, the use of mouthwash "Corsodil", which has powerful antiseptic properties (contains chlorhexidine in a concentration of 0.2%), contributes to an increase in pressure for several hours (by 2-3.5 units). This was established in an experiment involving 19 healthy volunteers (twice a day used the tool). The daily death of these small bacteria is a disaster, because increased pressure affects mortality from heart disease and stroke. So excessive oral hygiene can disrupt nitrate exchange.Stomach, nitrites, nitric oxide and nitrosamines.
In the stomach, two different processes involving nitrates and nitrites can occur. The direction of these processes depends on the acidity of the stomach. Consider both options.Normal (sufficient) stomach acidity of an adult.At such values of acidity there is no growth of bacteria that break down nitrates, i.e. the stomach of an adult, as a rule, has a too acidic environment to promote significant growth of such bacteria. The acidic environment works differently: part of the nitrite in saliva is converted to NO in the acidic environment of the stomach, and part is absorbed into the blood. Under certain conditions, this nitrite is converted to NO or other bioactive nitrogen oxides. The metabolism of nitrites in the stomach depending on the acidity of the gastric juice. Three aspects of the beneficial effect of nitric oxide in the stomach: antibacterial, mucus production and vascular expansion. They have a direct impact on the health of the stomach.
Low (physiological) acidity of the stomach.It happens normally in children and with stomach diseases in adults. Immediately after birth, the acidity of gastric juice is almost neutral and is about 6.0, after which it decreases to 1-2 pH units within 6-12 hours. However, by the end of the first week of life, the pH rises again to 5.0-6.0 and remains high for a long time, gradually decreasing to a pH of 3.0-4.0 by the end of the first year of life. At the age of 4-7 years, the total acidity index does not exceed 40 mmol / l, the pH on average is 2.5, in the future it decreases to the value of adults 1.5-2.0. With low acidity in the stomach, nitrites are not destroyed, but are absorbed into the blood in large enough quantities. In 1970, in Chile, it was found that the highest incidence of stomach cancer was observed in areas with high nitrate content in water and soil. Similar data were obtained in Japan, where a high incidence of stomach cancer was associated with the mutagenic effect of nitrates added to foods. Nitrates in the stomach cavity easily turn into nitrites, which, interacting with amines, form nitro compounds. Even small amounts of these substances have a strong carcinogenic effect on experimental animals. Don’t be in a hurry to blame nitrates! The key to understanding is the normal acidity of the stomach! The formation of nitro compounds is noticeably facilitated by increasing the pH (decrease in acidity) of the gastric contents. The nitrite/nitrate ratio at a pH above 5.0 is about 20 times greater than at a pH below 5.0. An increase in the content of nitrites in gastric juice during alkalinization of the medium appears to occur due to the development of nitrite-producing bacteria in the stomach; if in vitro studies add such bacteria to the gastric juice of healthy individuals, the number of nitro compounds in the juice will increase significantly. The formation of nitrites and therefore nitrozoamines is inhibited by vitamin C and the cooling of food. Comparative measurements of N-nitrose-containing substances in healthy people and patients with stomach diseases were carried out. With an increase in the pH of the stomach from 1 to 7, there was an increase in the content of N-nitroses. The highest level of these potential carcinogens was determined in patients with gastric carcinoma, with malignant anemia, as well as after partial gastrectomy. The theory that nitrozoamines are carcinogens explains the existence of areas with a high risk of stomach cancer. According to this theory, gastric cancer requires: food sources of nitrates, mechanisms for their recovery into nitrites, food sources of amines, as well as the blocking or absence of antagonists of these mutagens. In addition, nitric oxide in the stomach plays an important protective role. We have already found that in the acidic environment of the stomach, nitrite loses one oxygen atom, turning into a more active compound - nitric oxide, which is extremely toxic to many bacteria. Scientists have a logical question for this situation: are nitrates used to fight microbes? After all, nitrite, which got with saliva into the acidic environment of the stomach, can turn into nitric oxide and destroy microbes often present in food.
Experiments confirmed the scientists' guess: acidified saliva really kills E. coli. The only thing that was incomprehensible in this experiment was: how does nitrite in the mouth come from nitrate? It turned out that these transformations of one substance into another are performed by bacteria living on the back of the tongue, closer to the throat. That is, they to some extent protect us from many infectious gastric diseases.
Three aspects of the beneficial effect of nitric oxide in the stomach: antibacterial, mucus production and vascular expansion. They have a direct impact on the health of the stomach. Kidney excretion.Excess nitrates are easily excreted by the kidneys. This is more than 80% of the amount received.
Conclusion.Many vegetables contain high levels of nitrates, which can turn into nitrites. This amount is much higher than the nitrite content in meat. For example, a 125-gram serving of spinach can produce 881 mg of nitrates! The World Health Organization (WHO) calls the permissible daily dose of 3.7 mg of nitrates per 1 kg of body weight, and nitrites - 0.2 mg per kg. This refers to the nitrogen part of the salt: 250 mg of nitrates, safe for a conditional eater weighing 70 kg, are equivalent, for example, 350 mg of sodium nitrate. In different countries, the idea of the permissible dose of nitrates in the diet is different: in Germany it is 50-100 mg per day, in the United States - 400-500 mg, in most CIS countries - 300-320 mg. According to some researchers, the problem of nitrates is largely far-fetched, distracting consumers from the much more significant dangers of pesticides, chemical food additives (including nitrites) and other environmental problems. After all, the dose of nitrates that cause poisoning of the human body is much higher than the officially established limits (such cases are recorded with the simultaneous intake of nitrates in the form of mineral fertilizers in doses from 1 to 4 g). There are no studies that establish the relationship of exceeding the recommended consumption standards with the average life expectancy of people. Sodium nitrite is a necessary substance in the human body that protects against bacterial infections. Nitrites are produced by the human body independently, and also come with food. Sodium nitrite has vasodilating, bronchodilator properties, relieves spasms. Sodium nitrite preparations are used for angina pectoris, for spasms of cerebral vessels. Sodium nitrite is used as an antidote for cyanide poisoning.External source Vegetables, fruits, water, additives Preservative in meat and fish products Chemical industry, smoking, processed red meat Internal source The body itself synthesizes up to 25% of the total amount of nitrates Saliva bacteria (from nitrates), with reduced acidity - stomach bacteria. Excess nitrates with a lack of antioxidants in the acidic environment of the stomach Chemical activity in the body is quite inert, excess is easily excreted in the urine 30 times more active than nitrates, can oxidize hemoglobin Extremely dangerous even in small doses, carcinogenic compounds Use the source of nitric oxide, an important substance In the acidic environment of the stomach turns into nitric oxide. With low acidity and without AO turns into nitrosamines. For whom excess is especially dangerous for pregnant, lactating and children under 2 years old. Dangerous in foods with low antioxidants Dangerous for everyone always published
Author: Andrey Beloveshkin P.S. And remember, just by changing our consumption, we change the world together! © Join us on Facebook , VKontakte, Odnoklassniki
Source: beloveshkin.com/2016/01/udivitelnye-prevrashheniya-nitratov-v-organizme.html

After the body dies, nitrogen returns to the soil, and the cycle begins again. During this cycle, both nitrogen losses — when it is incorporated into sediments or released during the life of certain bacteria (called denitrifying bacteria) — and compensation for these losses through volcanic eruptions and other geological activities are possible. But most of the bound nitrogen man produces in the form of mineral fertilizers. Nitrogen deficiency often inhibits plant growth, and farmers buy artificially bound nitrogen in the form of mineral fertilizers to increase yields. Now for agriculture every year produces a little more than 80 million tons of bound nitrogen (note that it is used not only for growing food crops – suburban lawns and gardens fertilize them). Summarizing the entire human contribution to the nitrogen cycle, we get a figure of about 140 million tons per year. Approximately the same amount of nitrogen binds naturally in nature. Thus, in a relatively short period of time, man began to exert a significant influence on the nitrogen cycle in nature. What are the consequences? Each ecosystem is able to absorb a certain amount of nitrogen, and the consequences are generally favorable - plants will grow faster. However, when the ecosystem is saturated, nitrogen will begin to wash out into rivers and accumulate in excess in plants. But more on that later.
General characteristics of the nitrate pathway in the human body.Sources of nitrite intake in the stomach were studied on the example of the usual diet of a resident of the United States. The largest amount of dietary nitrates comes from vegetables - more than 85%. About 25% of food nitrates are involved in the cycle between the gut and saliva. Approximately 20% of nitrates under the influence of oral microflora are reduced to nitrites, which make up about 80% of all nitrites present in the contents of the stomach. Approximately 20% of nitrites are components of the daily diet. They found that the bulk of the nitrates contained in the eaten food products are absorbed in the digestive tract unchanged, enters the blood, and from it into the cells and tissues of the body. But within 6-8 hours the absorbed nitrates are almost completely excreted from the body by the kidneys. The remaining in the digestive tract, a smaller part of nitrates, indeed, with the help of microorganisms living in the digestive system, turns into nitrites. But these nitrites do not enter into undesirable reactions with the formation of carcinogenic substances - nitrosamines, as previously thought.

Nitric oxide (NO) is one of the strongest vasodilators and plays a key role in many significant processes – the regulation of vascular tone, pressure, platelet aggregation, mitochondrial respiration, nervous regulation and a number of others. NO synthesis is carried out in two main ways – related to the activity of the special enzyme NO synthase (NOS) and independent of the activity of NO synthase method. In addition to the intake of nitrates from food, they are also endogenously formed in the body during the oxidation of NO. The normal level of nitrates in the blood is 20-40 μM, and the level of nitrites is much lower - 50-1000 nM. The half-life of circulating nitrates in the blood is about 5 hours. It is still unclear how nitrates are actively absorbed by the salivary glands and their concentration in saliva is 20 times higher than in the blood. In the oral cavity, optional anaerobic bacteria restore nitrates to nitrites using the nitrate reductase enzyme.

Oral cavity, nitrates and nitrites.A significant amount of nitrates that are absorbed in the intestine enters the blood and is excreted with saliva. At the same time, the process of isolation goes not only during meals, but also in other hours. The transformation of nitrates primarily occurs in the oral cavity. Among the bacteria that inhabit the oral cavity, there are species that are able to restore nitric acid salts first to nitrite, and then to ammonium cation, through the formation of a number of intermediate products. That is, in the oral cavity there are microorganisms that produce enzymes nitratereductase and nitritreductase. The main producer of nitreductase in the oral cavity are bacteria of the genus Veillonella spp. Representatives of this kind of anaerobic bacteria dispose of lactic and other organic acids formed by streptococci, lactobacilli, yeast and actinomycetes, counteract the displacement of pH in the acid side due to lactic acid fermentation. That is, the detection in the oral cavity of Waylonella is a prognostically good sign. It is possible that with the risk of caries, the activity of nitreductase decreases. Studying the fate of nitrates in the human body, British biochemists have found that up to a quarter of all nitrates eaten with blood return to the cells of the oral cavity and are released into saliva. Here they turn into nitrites and saliva enter the digestive tract. Approximately 20% of nitrates under the influence of oral microflora are reduced to nitrites, which make up about 80% of all nitrites present in the contents of the stomach. Surprisingly, the use of mouthwash "Corsodil", which has powerful antiseptic properties (contains chlorhexidine in a concentration of 0.2%), contributes to an increase in pressure for several hours (by 2-3.5 units). This was established in an experiment involving 19 healthy volunteers (twice a day used the tool). The daily death of these small bacteria is a disaster, because increased pressure affects mortality from heart disease and stroke. So excessive oral hygiene can disrupt nitrate exchange.Stomach, nitrites, nitric oxide and nitrosamines.
In the stomach, two different processes involving nitrates and nitrites can occur. The direction of these processes depends on the acidity of the stomach. Consider both options.Normal (sufficient) stomach acidity of an adult.At such values of acidity there is no growth of bacteria that break down nitrates, i.e. the stomach of an adult, as a rule, has a too acidic environment to promote significant growth of such bacteria. The acidic environment works differently: part of the nitrite in saliva is converted to NO in the acidic environment of the stomach, and part is absorbed into the blood. Under certain conditions, this nitrite is converted to NO or other bioactive nitrogen oxides. The metabolism of nitrites in the stomach depending on the acidity of the gastric juice. Three aspects of the beneficial effect of nitric oxide in the stomach: antibacterial, mucus production and vascular expansion. They have a direct impact on the health of the stomach.
Low (physiological) acidity of the stomach.It happens normally in children and with stomach diseases in adults. Immediately after birth, the acidity of gastric juice is almost neutral and is about 6.0, after which it decreases to 1-2 pH units within 6-12 hours. However, by the end of the first week of life, the pH rises again to 5.0-6.0 and remains high for a long time, gradually decreasing to a pH of 3.0-4.0 by the end of the first year of life. At the age of 4-7 years, the total acidity index does not exceed 40 mmol / l, the pH on average is 2.5, in the future it decreases to the value of adults 1.5-2.0. With low acidity in the stomach, nitrites are not destroyed, but are absorbed into the blood in large enough quantities. In 1970, in Chile, it was found that the highest incidence of stomach cancer was observed in areas with high nitrate content in water and soil. Similar data were obtained in Japan, where a high incidence of stomach cancer was associated with the mutagenic effect of nitrates added to foods. Nitrates in the stomach cavity easily turn into nitrites, which, interacting with amines, form nitro compounds. Even small amounts of these substances have a strong carcinogenic effect on experimental animals. Don’t be in a hurry to blame nitrates! The key to understanding is the normal acidity of the stomach! The formation of nitro compounds is noticeably facilitated by increasing the pH (decrease in acidity) of the gastric contents. The nitrite/nitrate ratio at a pH above 5.0 is about 20 times greater than at a pH below 5.0. An increase in the content of nitrites in gastric juice during alkalinization of the medium appears to occur due to the development of nitrite-producing bacteria in the stomach; if in vitro studies add such bacteria to the gastric juice of healthy individuals, the number of nitro compounds in the juice will increase significantly. The formation of nitrites and therefore nitrozoamines is inhibited by vitamin C and the cooling of food. Comparative measurements of N-nitrose-containing substances in healthy people and patients with stomach diseases were carried out. With an increase in the pH of the stomach from 1 to 7, there was an increase in the content of N-nitroses. The highest level of these potential carcinogens was determined in patients with gastric carcinoma, with malignant anemia, as well as after partial gastrectomy. The theory that nitrozoamines are carcinogens explains the existence of areas with a high risk of stomach cancer. According to this theory, gastric cancer requires: food sources of nitrates, mechanisms for their recovery into nitrites, food sources of amines, as well as the blocking or absence of antagonists of these mutagens. In addition, nitric oxide in the stomach plays an important protective role. We have already found that in the acidic environment of the stomach, nitrite loses one oxygen atom, turning into a more active compound - nitric oxide, which is extremely toxic to many bacteria. Scientists have a logical question for this situation: are nitrates used to fight microbes? After all, nitrite, which got with saliva into the acidic environment of the stomach, can turn into nitric oxide and destroy microbes often present in food.
Experiments confirmed the scientists' guess: acidified saliva really kills E. coli. The only thing that was incomprehensible in this experiment was: how does nitrite in the mouth come from nitrate? It turned out that these transformations of one substance into another are performed by bacteria living on the back of the tongue, closer to the throat. That is, they to some extent protect us from many infectious gastric diseases.
Three aspects of the beneficial effect of nitric oxide in the stomach: antibacterial, mucus production and vascular expansion. They have a direct impact on the health of the stomach. Kidney excretion.Excess nitrates are easily excreted by the kidneys. This is more than 80% of the amount received.

Conclusion.Many vegetables contain high levels of nitrates, which can turn into nitrites. This amount is much higher than the nitrite content in meat. For example, a 125-gram serving of spinach can produce 881 mg of nitrates! The World Health Organization (WHO) calls the permissible daily dose of 3.7 mg of nitrates per 1 kg of body weight, and nitrites - 0.2 mg per kg. This refers to the nitrogen part of the salt: 250 mg of nitrates, safe for a conditional eater weighing 70 kg, are equivalent, for example, 350 mg of sodium nitrate. In different countries, the idea of the permissible dose of nitrates in the diet is different: in Germany it is 50-100 mg per day, in the United States - 400-500 mg, in most CIS countries - 300-320 mg. According to some researchers, the problem of nitrates is largely far-fetched, distracting consumers from the much more significant dangers of pesticides, chemical food additives (including nitrites) and other environmental problems. After all, the dose of nitrates that cause poisoning of the human body is much higher than the officially established limits (such cases are recorded with the simultaneous intake of nitrates in the form of mineral fertilizers in doses from 1 to 4 g). There are no studies that establish the relationship of exceeding the recommended consumption standards with the average life expectancy of people. Sodium nitrite is a necessary substance in the human body that protects against bacterial infections. Nitrites are produced by the human body independently, and also come with food. Sodium nitrite has vasodilating, bronchodilator properties, relieves spasms. Sodium nitrite preparations are used for angina pectoris, for spasms of cerebral vessels. Sodium nitrite is used as an antidote for cyanide poisoning.External source Vegetables, fruits, water, additives Preservative in meat and fish products Chemical industry, smoking, processed red meat Internal source The body itself synthesizes up to 25% of the total amount of nitrates Saliva bacteria (from nitrates), with reduced acidity - stomach bacteria. Excess nitrates with a lack of antioxidants in the acidic environment of the stomach Chemical activity in the body is quite inert, excess is easily excreted in the urine 30 times more active than nitrates, can oxidize hemoglobin Extremely dangerous even in small doses, carcinogenic compounds Use the source of nitric oxide, an important substance In the acidic environment of the stomach turns into nitric oxide. With low acidity and without AO turns into nitrosamines. For whom excess is especially dangerous for pregnant, lactating and children under 2 years old. Dangerous in foods with low antioxidants Dangerous for everyone always published
Author: Andrey Beloveshkin P.S. And remember, just by changing our consumption, we change the world together! © Join us on Facebook , VKontakte, Odnoklassniki
Source: beloveshkin.com/2016/01/udivitelnye-prevrashheniya-nitratov-v-organizme.html