483
Modeling photosynthesis
When I think about the genius of Nature (whoever and whatever the meaning is not invested in the word Nature), I want to say that her crown is a mankind individuals who proudly put the laptop on the belly, do what a few dozen years could only dream of: communicate with the world!
But from an environmental point of view, our appearance – only concument one of the highest orders, resting on the laurels of those who create biomass from non-life, i.e. on the laurels of our brethren autotrophic plants.
Notice that the vast majority of plants is not just the autotrophs and photoautotrophs, i.e. for the synthesis of organic compounds from inorganic ones using energy photons, the source of which is the Sun. It is not surprising that attempts not just to reproduce the process of photosynthesis, but to surpass it and put it in a big way has received a lot of attention from scientists.
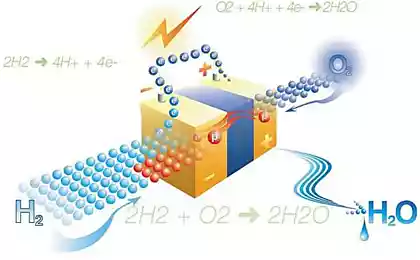
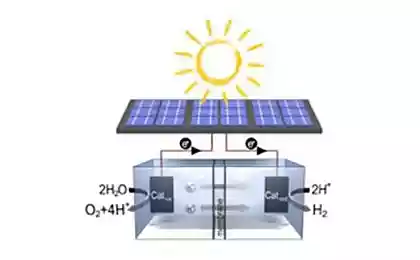
As you know, the byproduct of photosynthesis is oxygen produced during the oxidation of water under the action of photosystem II (PSII). Briefly and simplistically, let me remind you how it works.
A quantum of light hits the chlorophyll and knock him out of the electron. This electron then eventually ends up in photosystem I, but deprived of its chlorophyll and became a strong oxidant, takes through brex into voodoochilli complex (FOC) electrons from water, with the result that oxygen is produced.
Sixty three million four hundred forty five thousand two hundred sixty three
Thus, the SAI can be seen as a catalyst for the oxidation of water. It is the imitation of this part of the FS II researchers are very active.
I must say that the potential (i.e., thermodynamic) water to oxidize any oxidizer, whose electrode potential above its electrode potential. For example, potassium permanganate (E°=+1.51 In for the half-reaction MnO4-+5e-+8H+ → Mn2+ +4H2O). You can look at the table of standard electrode potentials and to make sure that there are other examples. However, in practice this does not happen for kinetic reasons, in other words, due to the high activation energy of the rate of this process is very small. Therefore, development of a catalyst for the oxidation of water is relevant, and biomimetic approach is promising.
In homogeneous catalysis (i.e., catalytic reactions where the catalyst is in the same phase as the reagents, in practice, mainly in the liquid), the catalyst activity can be evaluated in such a setting as "speed" (TOF, turnover frequency), i.e. the number of molecules of reactant converted to one molecule of catalyst (more precisely – the active centre) in unit time has the dimension s-1. Wok PSII has a TOF of about 100-400 s-1.
Researchers from the University of würzburg as a catalyst for the oxidation of water decided to use a complex of ruthenium containing 3 atom of this element [Ru(bda)bpb]3.
"Why ruthenium?"you ask and I answer: probably because the set of oxidation States of this element (+2, +3, + 4, +5) painfully reminiscent of the set of degrees of oxidation of manganese, which, as the researchers believe, accept his atoms in PT in the oxidation of water.
Eighty seven million seventy four thousand six hundred seventy
That is able to do this noble handsome?
In water-acetonitrile mixture at pH=1, it is able to catalyze the oxidation of water by the ammonium nitrate-cerium (IV) (E°=+1,72 In for the half-reaction Ce4++e- → Ce3+). As soon as this strong oxidising agent is added to the system containing a small amount of catalyst, it immediately starts bubbling of oxygen, whose concentration in the gas phase above the solution increases dramatically! The TOF of this catalyst is close to the efficiency of natural wok and is about 160 s-1. Proceeds reaction: 2Ce4+ + H2O → 2Ce3+ + 1/2O2 + 2H+.
Fifty three million six hundred sixty seven thousand three hundred five
However, scientists did not stop there. The researchers decided to design a system that would work photochemically, i.e. in some way imitated the work of FS II. The second key player this biomimetic design was another complex of ruthenium as a photosensitizer. Here's how it works.
Eighty seven million six hundred forty six thousand two hundred fifty four
A photon (shown as lightning bolt) knocks out an electron from a photosensitizer (a circle of red arrows). Electron "goes left", to an external acceptor, peroxodisulfate sodium (E°=+2,01 In for the half-reaction S2O82-+2e- → 2SO42-), and the hole, which in our case is represented by the ruthenium atom with oxidation state (+3) oxidizes the catalyst (the circle of blue arrows), which in turn takes an electron from water. Thus, the total equation of the proceeding reactions is: S2O82-+H2O → 2SO42- + 1/2O2 + 2H+.
What is the advantage created by German researchers of a catalyst?
1) He is very active (enters a very small and elite group of catalysts capable of achieving TOFs in excess of 100 s–1). In photochemical process oxygen significantly already at a concentration of catalyst of about 90 nm, i.e. 90×10-9 mol/L.
2) due to the fact that the catalytically active ruthenium atoms are strongly bound like a fly in a web, polydentate ligand, complex catalyst monetarnih their more stable counterparts.
The stability of the catalyst is characterized by such a parameter as "speed" (TON, turnover number) is the number of catalytic cycles that can crank active site until the deactivation (termination of work). In oxidation reaction of water under the action of Ce(IV), TON is for him about 7400 vs 1000 for monetarnih counterparts. However, in the case of photochemical process TON lower (less resistance) is about 1200.
Well, about the faults.
Article about the discovery of a new catalyst printed in the journal of family Nature (Nature Chemistry), which publishes advanced and most important for the chemical community and, presumably, for humanity work and achievement (impact factor for 2014 is 25.3).
So. Everything to date is able to make mankind, is not the cheapest metal of ruthenium (in the nature of work cheap manganese) in 0.1 n sulfuric acid (pH=1, about the same acidity, just below the stomach; the oxidation of water in nature is at a pH close to 7) and 60 % acetonitrile (organic solvent, which does not require chloroplasts) to give dozens micromole oxygen per second. But there is something to strive for! published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/275460/
But from an environmental point of view, our appearance – only concument one of the highest orders, resting on the laurels of those who create biomass from non-life, i.e. on the laurels of our brethren autotrophic plants.
Notice that the vast majority of plants is not just the autotrophs and photoautotrophs, i.e. for the synthesis of organic compounds from inorganic ones using energy photons, the source of which is the Sun. It is not surprising that attempts not just to reproduce the process of photosynthesis, but to surpass it and put it in a big way has received a lot of attention from scientists.
As you know, the byproduct of photosynthesis is oxygen produced during the oxidation of water under the action of photosystem II (PSII). Briefly and simplistically, let me remind you how it works.
A quantum of light hits the chlorophyll and knock him out of the electron. This electron then eventually ends up in photosystem I, but deprived of its chlorophyll and became a strong oxidant, takes through brex into voodoochilli complex (FOC) electrons from water, with the result that oxygen is produced.
Sixty three million four hundred forty five thousand two hundred sixty three
Thus, the SAI can be seen as a catalyst for the oxidation of water. It is the imitation of this part of the FS II researchers are very active.
I must say that the potential (i.e., thermodynamic) water to oxidize any oxidizer, whose electrode potential above its electrode potential. For example, potassium permanganate (E°=+1.51 In for the half-reaction MnO4-+5e-+8H+ → Mn2+ +4H2O). You can look at the table of standard electrode potentials and to make sure that there are other examples. However, in practice this does not happen for kinetic reasons, in other words, due to the high activation energy of the rate of this process is very small. Therefore, development of a catalyst for the oxidation of water is relevant, and biomimetic approach is promising.
In homogeneous catalysis (i.e., catalytic reactions where the catalyst is in the same phase as the reagents, in practice, mainly in the liquid), the catalyst activity can be evaluated in such a setting as "speed" (TOF, turnover frequency), i.e. the number of molecules of reactant converted to one molecule of catalyst (more precisely – the active centre) in unit time has the dimension s-1. Wok PSII has a TOF of about 100-400 s-1.
Researchers from the University of würzburg as a catalyst for the oxidation of water decided to use a complex of ruthenium containing 3 atom of this element [Ru(bda)bpb]3.
"Why ruthenium?"you ask and I answer: probably because the set of oxidation States of this element (+2, +3, + 4, +5) painfully reminiscent of the set of degrees of oxidation of manganese, which, as the researchers believe, accept his atoms in PT in the oxidation of water.
Eighty seven million seventy four thousand six hundred seventy
That is able to do this noble handsome?
In water-acetonitrile mixture at pH=1, it is able to catalyze the oxidation of water by the ammonium nitrate-cerium (IV) (E°=+1,72 In for the half-reaction Ce4++e- → Ce3+). As soon as this strong oxidising agent is added to the system containing a small amount of catalyst, it immediately starts bubbling of oxygen, whose concentration in the gas phase above the solution increases dramatically! The TOF of this catalyst is close to the efficiency of natural wok and is about 160 s-1. Proceeds reaction: 2Ce4+ + H2O → 2Ce3+ + 1/2O2 + 2H+.
Fifty three million six hundred sixty seven thousand three hundred five
However, scientists did not stop there. The researchers decided to design a system that would work photochemically, i.e. in some way imitated the work of FS II. The second key player this biomimetic design was another complex of ruthenium as a photosensitizer. Here's how it works.
Eighty seven million six hundred forty six thousand two hundred fifty four
A photon (shown as lightning bolt) knocks out an electron from a photosensitizer (a circle of red arrows). Electron "goes left", to an external acceptor, peroxodisulfate sodium (E°=+2,01 In for the half-reaction S2O82-+2e- → 2SO42-), and the hole, which in our case is represented by the ruthenium atom with oxidation state (+3) oxidizes the catalyst (the circle of blue arrows), which in turn takes an electron from water. Thus, the total equation of the proceeding reactions is: S2O82-+H2O → 2SO42- + 1/2O2 + 2H+.
What is the advantage created by German researchers of a catalyst?
1) He is very active (enters a very small and elite group of catalysts capable of achieving TOFs in excess of 100 s–1). In photochemical process oxygen significantly already at a concentration of catalyst of about 90 nm, i.e. 90×10-9 mol/L.
2) due to the fact that the catalytically active ruthenium atoms are strongly bound like a fly in a web, polydentate ligand, complex catalyst monetarnih their more stable counterparts.
The stability of the catalyst is characterized by such a parameter as "speed" (TON, turnover number) is the number of catalytic cycles that can crank active site until the deactivation (termination of work). In oxidation reaction of water under the action of Ce(IV), TON is for him about 7400 vs 1000 for monetarnih counterparts. However, in the case of photochemical process TON lower (less resistance) is about 1200.
Well, about the faults.
Article about the discovery of a new catalyst printed in the journal of family Nature (Nature Chemistry), which publishes advanced and most important for the chemical community and, presumably, for humanity work and achievement (impact factor for 2014 is 25.3).
So. Everything to date is able to make mankind, is not the cheapest metal of ruthenium (in the nature of work cheap manganese) in 0.1 n sulfuric acid (pH=1, about the same acidity, just below the stomach; the oxidation of water in nature is at a pH close to 7) and 60 % acetonitrile (organic solvent, which does not require chloroplasts) to give dozens micromole oxygen per second. But there is something to strive for! published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/275460/