491
Who are threatened by osteoporosis
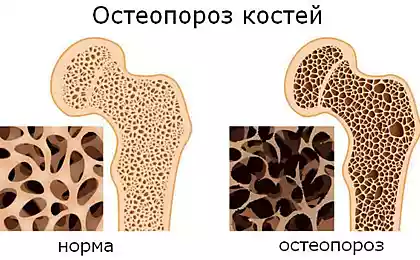
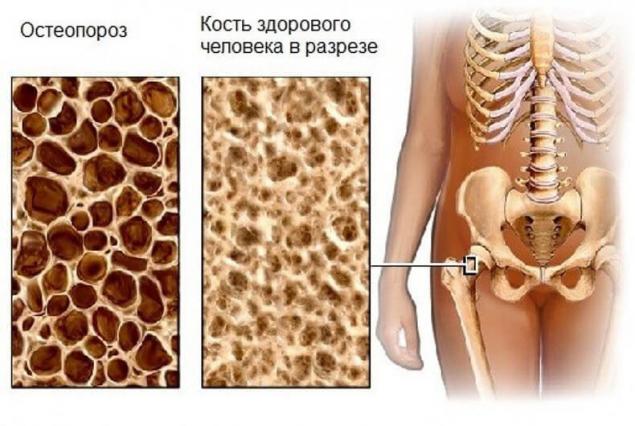
Osteoporosis is a systemic diseasethat affects all bones of the skeleton, accompanied by a decrease of the density and strength of bones, leading to a high risk of fractures even after minimal trauma such as falling from the height of your height or lifting weight of about 10 kg.
Many pseudo-scientists have linked the occurrence of osteoporosis from lack of calcium in the blood, which leads, in their opinion, insufficient consumption of dairy products. However... How then to explain the following fact?
If you go to the who website and look at the statistics for osteoporosis, you will see that on the first places in Europe and the United States, leading to the consumption of milk and meat. Completing the list of African countries where the protein is produced traditionally from soy, corn, legumes. In Africa traditionally do not consume dairy products, the majority of Africans to the steadfast intolerance!
The research of Dr. Helen Linkswiler from the University of Wisconsin showed a direct relationship of calcium loss from the bones resulting from the consumption of animal protein. Similar experiments are conducted and mark Hegsted from Harvard.
Daily consumption of 50 g of protein causes leaching of calcium from bones. While consumption of 95 g of the leaching also depends on the amount of phosphorus in your diet. Note that the measurements were carried out only on the results of the loss of calcium with urine, i.e. not taken into account the calcium that is deposited in plaques of cholesterol and gallstones and kidney.
Let us leave the protein and see what else affects the leaching of calcium from bones. Animal food contains approximately 10 times more phosphorus than the plant. A strong dependence of the loss of calcium from bone and excessive use of phosphorus. How does this mechanism work? For this we need to consider the topic of acid-base balance (ABB).
The term AAR is applied to aqueous solutions, liquids. As for the natural minerals, then they are hitting in the solution may be either acid-forming or locaabrace. There are trace elements that shift the blood pH to the alkaline side (calcium, potassium, magnesium, sodium) and elements that shift the balance to the acid side (sulfur, phosphorus, chlorine).
Our body maintains the acidity of each system, each organ in the strict boundaries, whereby there can not exist the majority of pathogenic microorganisms. Furthermore, there are constant fluctuations around a fixed value. So blood pH ranges from 7.35 to 7.45 during the day.

After this information let us recall that we consider the impact of food on blood acidity. Now imagine that in the blood phosphorus. It shifts the acid-base balance (ABB) in the acid side.
The body tries to compensate by throwing an calcium from their buffer stock from bones. Calcium binds phosphorus, the balance is restored. Chlorine and sulfur give the same effect as phosphorus. Potassium, magnesium and sodium have the same effect as the calcium, magnesium and sodium there is in the body in sufficient quantity. Potassium can not be a buffer, because its deficiency in cells would dramatically affect the viability of the organism. So use the calcium. Interestingly, this same mechanism also blocks the excretion of calcium through the kidneys, the body tries to maintain the buffer elements, and as a result, calcium salts are deposited in the kidneys.
To maintain ABB within the range characteristic of each species, he needs to eat the food that does not break the balance. Predators need to eat raw meat, offal and bones. Herbivore – grass and leaves. Man – vegetables, fruits, and herbs. And the greater the deviation from the CR unit, the more blood is shifted to the AAR, the more the body uses internal buffer resources to restore equilibrium.
See also: Your language is an indicator of the purity of the whole body
A powerful tool for treatment of joints
And since we're talking about osteoporosis (porous bones), the consumption of food of animal origin — uniquely contributes to the leaching of calcium from bones. With regard to incremental dietary intake of calcium, its absorption into the body is limited and slowed down, as soon as the entrance is found a lot of it.
Therefore, the admission of various gluconates and dairy products will lead to the opposite effect – all that is taken will go in the urine and stones, and the fact that you tried to compensate for reception, will be offset by the calcium from the bones. This is a paradox. published
Author: Galina Kislyakova
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: vk.com/probujdeniechelovechnost?w=wall-66683693_6680%2Fall
Many pseudo-scientists have linked the occurrence of osteoporosis from lack of calcium in the blood, which leads, in their opinion, insufficient consumption of dairy products. However... How then to explain the following fact?
If you go to the who website and look at the statistics for osteoporosis, you will see that on the first places in Europe and the United States, leading to the consumption of milk and meat. Completing the list of African countries where the protein is produced traditionally from soy, corn, legumes. In Africa traditionally do not consume dairy products, the majority of Africans to the steadfast intolerance!

The research of Dr. Helen Linkswiler from the University of Wisconsin showed a direct relationship of calcium loss from the bones resulting from the consumption of animal protein. Similar experiments are conducted and mark Hegsted from Harvard.
Daily consumption of 50 g of protein causes leaching of calcium from bones. While consumption of 95 g of the leaching also depends on the amount of phosphorus in your diet. Note that the measurements were carried out only on the results of the loss of calcium with urine, i.e. not taken into account the calcium that is deposited in plaques of cholesterol and gallstones and kidney.
Let us leave the protein and see what else affects the leaching of calcium from bones. Animal food contains approximately 10 times more phosphorus than the plant. A strong dependence of the loss of calcium from bone and excessive use of phosphorus. How does this mechanism work? For this we need to consider the topic of acid-base balance (ABB).
The term AAR is applied to aqueous solutions, liquids. As for the natural minerals, then they are hitting in the solution may be either acid-forming or locaabrace. There are trace elements that shift the blood pH to the alkaline side (calcium, potassium, magnesium, sodium) and elements that shift the balance to the acid side (sulfur, phosphorus, chlorine).
Our body maintains the acidity of each system, each organ in the strict boundaries, whereby there can not exist the majority of pathogenic microorganisms. Furthermore, there are constant fluctuations around a fixed value. So blood pH ranges from 7.35 to 7.45 during the day.

After this information let us recall that we consider the impact of food on blood acidity. Now imagine that in the blood phosphorus. It shifts the acid-base balance (ABB) in the acid side.
The body tries to compensate by throwing an calcium from their buffer stock from bones. Calcium binds phosphorus, the balance is restored. Chlorine and sulfur give the same effect as phosphorus. Potassium, magnesium and sodium have the same effect as the calcium, magnesium and sodium there is in the body in sufficient quantity. Potassium can not be a buffer, because its deficiency in cells would dramatically affect the viability of the organism. So use the calcium. Interestingly, this same mechanism also blocks the excretion of calcium through the kidneys, the body tries to maintain the buffer elements, and as a result, calcium salts are deposited in the kidneys.
To maintain ABB within the range characteristic of each species, he needs to eat the food that does not break the balance. Predators need to eat raw meat, offal and bones. Herbivore – grass and leaves. Man – vegetables, fruits, and herbs. And the greater the deviation from the CR unit, the more blood is shifted to the AAR, the more the body uses internal buffer resources to restore equilibrium.
See also: Your language is an indicator of the purity of the whole body
A powerful tool for treatment of joints
And since we're talking about osteoporosis (porous bones), the consumption of food of animal origin — uniquely contributes to the leaching of calcium from bones. With regard to incremental dietary intake of calcium, its absorption into the body is limited and slowed down, as soon as the entrance is found a lot of it.
Therefore, the admission of various gluconates and dairy products will lead to the opposite effect – all that is taken will go in the urine and stones, and the fact that you tried to compensate for reception, will be offset by the calcium from the bones. This is a paradox. published
Author: Galina Kislyakova
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: vk.com/probujdeniechelovechnost?w=wall-66683693_6680%2Fall