509
4 incredible chemical substance, open people
Scientists are discovering and creating new material constantly. But sometimes found things that the jaw comes down with a crash on the floor. They need to know in person. From the indescribable light solids used by NASA, to metals which melt in the hand, we have found some incredible chemicals, known and not. Quickly it will run.
Aerogel: the lightest solid
This amazing gel is the most easy in the world solid. Since its invention in 1931 by the American scholar Samuel Killerom, it was used in space missions to collect dust from comet state agencies for the development of insulated tents and even for making clothing that protects a person from extreme heat.
NASA called it "blue smoke" because it looks like a hologram.
Cool this substance make it paradoxical properties. This hard gel consists mostly of air, so it weighs very little, resembling a sponge. While it is perfectly repels heat. As you can see in the picture below, it protects the flower from the flames.

Individual molecules that make up the aerogel, act like miniature baseball glove — they catch fast-moving particles without damaging them. This property was extremely useful during the NASA Stardust mission.
Scientists have filled a massive silicate aerogel collector in the form of the racket, which was outside on the ship Stardust. His goal was to capture the fragile particles remaining after the comet Wilde-2 without damaging them. Because the aerogel solid and relatively transparent, the scientists easily found and extracted particles for later analysis.
The precursor aerogel structurally resembles jelly. Gelatin powder in jelly forms a flexible, liquid solution when mixed with warm water, after which cooled to a hard complicated network, which chemically resembles the naughty ball into the harness, taking the prescribed form. If you heat the jelly, it will dry and you'll still get the powder.
Aerogel, on the other hand, does not consist of gelatin. It is most often made from silicon, the most common mineral in the crust of the Earth. Wet aerogel goes through a cycle of cooling and heating under pressure, allowing it to retain its shape even after drying. The resulting aerogel is almost air, solid and very light. To the touch it is like Styrofoam. Aerogel you can even do it yourself if you know how.
Gallium: metal that melts at room temperature
This soft, lustrous and hard metal at the same time is quite unusual. At low temperatures it takes a solid form. But upon heating to room temperature it melts into a glorious puddle.

Until now its primary use was in the production of smartphones, the aerospace industry and in the field of communication. Although this chemical element is present in the periodic table in nature, it is not found. Its traces can be found in zinc ore and bauxite, which makes aluminum. Still available on Amazon, where can I buy just 10 bucks.
And if you manage to get hold of, keep it away from the technique — it is bad for other metals. This will be especially noticeable if the aluminium on the back of your phone scratched, allowing Gaul to penetrate deeper into the metal lattice. Look what happens if you throw gallium scratched the cover iPhone.
After a few hours it is completely decomposed.

Diamond nanowires: a possible basis for the space Elevator?
This is a relatively new man-made fiber made from carbon atoms arranged in a zigzag structure, similar to diamond, may be the most durable and hard material we've ever done.
Opened in 2014, this fiber showed a strength, which is superior to carbon nanotubes, another ultra-strong and lightweight material. With all this, it is extremely thin. Only three atoms in diameter, much thinner than a human hair. Since this structure was opened recently, its composition must still confirm high resolution images.

The properties and behaviour also need a deeper understanding before it can be produced in commercial scale. But if it works, these diamond nanofibers can in theory be strong enough to form the basis of the cable for the space Elevator. Other candidates, such as steel, break under its own weight.
Ferromagnetic fluid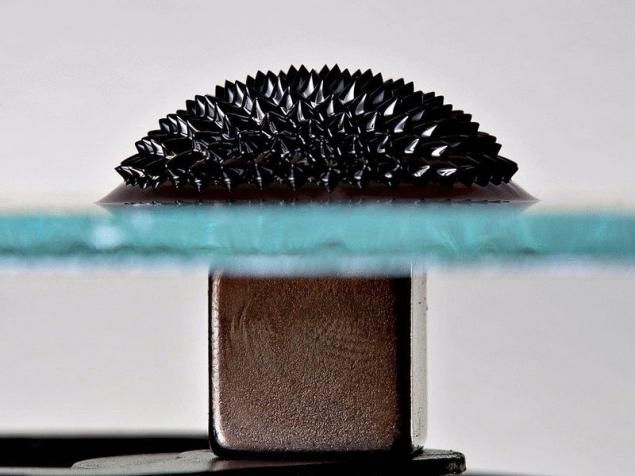
This is similar to the porcupine a bunch of ultrafine magnetic particles of iron, generally is a liquid which begins to dance and build incredible structures when exposed to a magnetic field.
Each tiny particle in ferrofluid (ferrofluid) coated with surfactant, which prevents the sticking of particles together, and suspendirovanie in liquid — water, for example. These particles are not like the magnets on your fridge. This is the "paramagnetic" particles, that is, become tiny magnets in the presence of a magnetic field, which move and coalesce with other tiny magnets in the field.

Ferromagnetic fluid was established in 1963 NASA scientist Steve Pappilon as a prototype for the rocket fuel that was to propel the spacecraft after the application of a magnetic field on it. The strangest thing in ferromagnetic fluids that they behave both as liquid and as solid materials.published
P. S. And remember, just changing your mind — together we change the world! ©
Source: hi-news.ru
Aerogel: the lightest solid

This amazing gel is the most easy in the world solid. Since its invention in 1931 by the American scholar Samuel Killerom, it was used in space missions to collect dust from comet state agencies for the development of insulated tents and even for making clothing that protects a person from extreme heat.
NASA called it "blue smoke" because it looks like a hologram.
Cool this substance make it paradoxical properties. This hard gel consists mostly of air, so it weighs very little, resembling a sponge. While it is perfectly repels heat. As you can see in the picture below, it protects the flower from the flames.

Individual molecules that make up the aerogel, act like miniature baseball glove — they catch fast-moving particles without damaging them. This property was extremely useful during the NASA Stardust mission.
Scientists have filled a massive silicate aerogel collector in the form of the racket, which was outside on the ship Stardust. His goal was to capture the fragile particles remaining after the comet Wilde-2 without damaging them. Because the aerogel solid and relatively transparent, the scientists easily found and extracted particles for later analysis.
The precursor aerogel structurally resembles jelly. Gelatin powder in jelly forms a flexible, liquid solution when mixed with warm water, after which cooled to a hard complicated network, which chemically resembles the naughty ball into the harness, taking the prescribed form. If you heat the jelly, it will dry and you'll still get the powder.
Aerogel, on the other hand, does not consist of gelatin. It is most often made from silicon, the most common mineral in the crust of the Earth. Wet aerogel goes through a cycle of cooling and heating under pressure, allowing it to retain its shape even after drying. The resulting aerogel is almost air, solid and very light. To the touch it is like Styrofoam. Aerogel you can even do it yourself if you know how.
Gallium: metal that melts at room temperature

This soft, lustrous and hard metal at the same time is quite unusual. At low temperatures it takes a solid form. But upon heating to room temperature it melts into a glorious puddle.

Until now its primary use was in the production of smartphones, the aerospace industry and in the field of communication. Although this chemical element is present in the periodic table in nature, it is not found. Its traces can be found in zinc ore and bauxite, which makes aluminum. Still available on Amazon, where can I buy just 10 bucks.
And if you manage to get hold of, keep it away from the technique — it is bad for other metals. This will be especially noticeable if the aluminium on the back of your phone scratched, allowing Gaul to penetrate deeper into the metal lattice. Look what happens if you throw gallium scratched the cover iPhone.
After a few hours it is completely decomposed.

Diamond nanowires: a possible basis for the space Elevator?

This is a relatively new man-made fiber made from carbon atoms arranged in a zigzag structure, similar to diamond, may be the most durable and hard material we've ever done.
Opened in 2014, this fiber showed a strength, which is superior to carbon nanotubes, another ultra-strong and lightweight material. With all this, it is extremely thin. Only three atoms in diameter, much thinner than a human hair. Since this structure was opened recently, its composition must still confirm high resolution images.

The properties and behaviour also need a deeper understanding before it can be produced in commercial scale. But if it works, these diamond nanofibers can in theory be strong enough to form the basis of the cable for the space Elevator. Other candidates, such as steel, break under its own weight.
Ferromagnetic fluid

This is similar to the porcupine a bunch of ultrafine magnetic particles of iron, generally is a liquid which begins to dance and build incredible structures when exposed to a magnetic field.
Each tiny particle in ferrofluid (ferrofluid) coated with surfactant, which prevents the sticking of particles together, and suspendirovanie in liquid — water, for example. These particles are not like the magnets on your fridge. This is the "paramagnetic" particles, that is, become tiny magnets in the presence of a magnetic field, which move and coalesce with other tiny magnets in the field.

Ferromagnetic fluid was established in 1963 NASA scientist Steve Pappilon as a prototype for the rocket fuel that was to propel the spacecraft after the application of a magnetic field on it. The strangest thing in ferromagnetic fluids that they behave both as liquid and as solid materials.published
P. S. And remember, just changing your mind — together we change the world! ©
Source: hi-news.ru