977
German scientists have measured the mass of the electron is 13 times more precisely than ever before
Electron - the first known person to elementary particles. It was opened in 1897 by the British physicist Joseph John Thompson. Unlike many other particles that were previously submitted by elementary, and then were composed of other particles, the electron is still considered one of the fundamental building blocks of the universe, and its mass and electric charge - one of the fundamental physical constants. Therefore, an accurate measurement of its mass is of great interest. Recently, a team of German physicists managed to break the record of accuracy of measuring the mass of an electron.

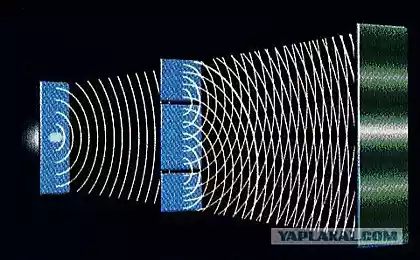
Electron - light particle and measure its mass directly is very difficult. The scientists used a highly ionized carbon atom, which has only one electron. Carbon Ion was imprisoned in ловушку Penning (pictured) - a device for the storage of charged particles in a uniform magnetic field. Measuring the performance of the system, consisting of a nucleus of an atom of carbon and one electron, the scientists were able to very accurately determine the ratio of the mass of the electron to the nucleus of carbon-12. The mass of the carbon nucleus is very well known - it is a kind of benchmark: atomic mass unit is by definition equal to one-twelfth the mass of the nucleus of an atom of carbon.
The specified value of the electron mass - 0.000548579909067 amu Usually increase the accuracy of measuring the mass of elementary particles on the order achieved 10 - 20 years. At this time, the accuracy was increased by 13 times in just a few years after the previous record accurately measure the mass of the electron.
Article describing the experiment опубликована in the journal Nature. Пресс-релиз Institute of Nuclear Physics, Max Planck Society at the University of Heidelberg (in German).
Source: habrahabr.ru/post/217609/

Electron - light particle and measure its mass directly is very difficult. The scientists used a highly ionized carbon atom, which has only one electron. Carbon Ion was imprisoned in ловушку Penning (pictured) - a device for the storage of charged particles in a uniform magnetic field. Measuring the performance of the system, consisting of a nucleus of an atom of carbon and one electron, the scientists were able to very accurately determine the ratio of the mass of the electron to the nucleus of carbon-12. The mass of the carbon nucleus is very well known - it is a kind of benchmark: atomic mass unit is by definition equal to one-twelfth the mass of the nucleus of an atom of carbon.
The specified value of the electron mass - 0.000548579909067 amu Usually increase the accuracy of measuring the mass of elementary particles on the order achieved 10 - 20 years. At this time, the accuracy was increased by 13 times in just a few years after the previous record accurately measure the mass of the electron.
Article describing the experiment опубликована in the journal Nature. Пресс-релиз Institute of Nuclear Physics, Max Planck Society at the University of Heidelberg (in German).
Source: habrahabr.ru/post/217609/