429
Of sodium and carbon created by the electrodes with enhanced conductivity
A team of scientists from Michigan technological University have created a completely new way of synthesizing carbon nanostruc interspersed with sodium. Until now this material existed only in theory.The inclusion of sodium in carbon materials can greatly improve the electrodes, which will lead to the modernization of production of solar cells and supercapacitors.
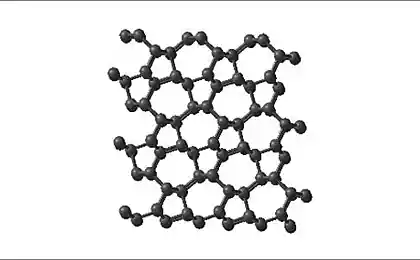
High conductivity and large surface area required for ideal electrodes, in modern materials confront each other. Amorphous carbon low conductivity, but a large surface area. In graphite, on the other hand, high conductivity, but low surface area. Three-dimensional graphite takes them all the best, and the carbon with sodium and surpasses it.
"Conductivity of carbon inclusions of sodium is two orders of magnitude more than the three-dimensional graphene, says Professor Yunhan Hu, the head of research. — Structure naostanok, with all their channels and pores also has a large surface, compared to graphene".

For the production of this material dream, Hu and his team have developed a new process. They used a temperature-controlled reaction between sodium and carbon monoxide to create carbon black powder, which picks up the sodium atoms. As a result, the sodium penetrated into the carbon, and not remain on its surface.
Only a small amount of sodium is required in order for the acquired carbon properties of high conductivity. Thus, this material can become the basis for the production of electrodes in solar cells and supercapacitors.
Flexible supercapacitors that are able to be recharged more than 30 thousand of times without performance degradation, has been developed by scientists at the University of Central Florida. This method is able to make a technological revolution in several spheres, from mobile phones to electric cars. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hightech.fm/2016/12/21/sodium-nanomaterial

High conductivity and large surface area required for ideal electrodes, in modern materials confront each other. Amorphous carbon low conductivity, but a large surface area. In graphite, on the other hand, high conductivity, but low surface area. Three-dimensional graphite takes them all the best, and the carbon with sodium and surpasses it.
"Conductivity of carbon inclusions of sodium is two orders of magnitude more than the three-dimensional graphene, says Professor Yunhan Hu, the head of research. — Structure naostanok, with all their channels and pores also has a large surface, compared to graphene".

For the production of this material dream, Hu and his team have developed a new process. They used a temperature-controlled reaction between sodium and carbon monoxide to create carbon black powder, which picks up the sodium atoms. As a result, the sodium penetrated into the carbon, and not remain on its surface.
Only a small amount of sodium is required in order for the acquired carbon properties of high conductivity. Thus, this material can become the basis for the production of electrodes in solar cells and supercapacitors.
Flexible supercapacitors that are able to be recharged more than 30 thousand of times without performance degradation, has been developed by scientists at the University of Central Florida. This method is able to make a technological revolution in several spheres, from mobile phones to electric cars. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hightech.fm/2016/12/21/sodium-nanomaterial
Acura will remove the personal videos of each released hybrid supercar NSX
Racing electric car Tesla Model S P100D is faster Formula 1