479
A new step in genetic medicine
A drug that corrects errors in the human genetic code, was first approved for use in EU countries on a commercial basis. The drug called Glybera (Glybera) has received permission from the European Commission and can now be sold in all pharmacies in the EU.
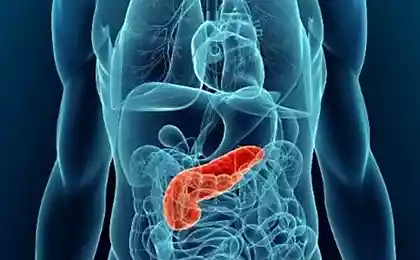
The drug was developed to treat pancreatitis – a rare disease which makes the body to properly process fats. Manufacturers say that the drug will already be available for purchase next year.
The principle of gene therapy is very simple: if a part of the human genetic code there is a problem, the medication just replaces it to work. However, some studies had problems: several patients from the US have developed leukemia.

Glybera used to treat deficiency of lipoprotein lipase. People with a damaged copy of the gene that is responsible for the breakdown of fats and the production of lipoprotein lipase, exist in the world, only one patient in a million. The inability to break down fats causes the fat accumulates in the blood. It causes abdominal pain and life-threatening inflammation of the pancreas (pancreatitis).
Until recently, the only treatment for this disease was a diet extremely low in fat.
Therapy, developed by UniQure, uses a special virus that migrates to the cells of the body, the working copy of the gene. The European medicines Agency has approved this therapy for treating the most critically ill patients in the beginning of this year.
Executive Director of UniQure Jorn Aldag said, "Final approval of Glybera the European Commission is a very important step for pharmacology and medicine, because it makes possible further application of gene therapy for the treatment of other rare and not amenable to traditional drugs in disease"
The company also announced that it intends to promote their drug for approval in the U.S. and Canada.

The first country in the world that has approved a gene therapy, was China. It happened in 2003, when the company Shenzhen SiBiono GenTech obtained a license of the State administration for food and drug administration of China for the commercial use of recombinant gene therapy Ad-p53 for the treatment of squamous cell carcinoma of the head and neck. This type of cancer accounts for about 10% of the 2.5 million new cancer patients in China.
After five years of clinical tests, Chinese researchers came to very good results. The only side effect of Ad-p53 is a fever, but not all patients. After eight weeks of joint use of radiation and gene therapy 64% of patients with advanced tumors showed complete regression and 32% partial.
The example of China shows that gene therapy could be a real ambrosia in modern medicine. As they say, many researchers in China, the procedure for obtaining a license for medicines is much easier than in Europe and the United States. But since in 2012 "caved in" and Europe, it is quite possible that the dominance of gene therapies is not far off.
Source: /users/104
The drug was developed to treat pancreatitis – a rare disease which makes the body to properly process fats. Manufacturers say that the drug will already be available for purchase next year.
The principle of gene therapy is very simple: if a part of the human genetic code there is a problem, the medication just replaces it to work. However, some studies had problems: several patients from the US have developed leukemia.

Glybera used to treat deficiency of lipoprotein lipase. People with a damaged copy of the gene that is responsible for the breakdown of fats and the production of lipoprotein lipase, exist in the world, only one patient in a million. The inability to break down fats causes the fat accumulates in the blood. It causes abdominal pain and life-threatening inflammation of the pancreas (pancreatitis).
Until recently, the only treatment for this disease was a diet extremely low in fat.
Therapy, developed by UniQure, uses a special virus that migrates to the cells of the body, the working copy of the gene. The European medicines Agency has approved this therapy for treating the most critically ill patients in the beginning of this year.
Executive Director of UniQure Jorn Aldag said, "Final approval of Glybera the European Commission is a very important step for pharmacology and medicine, because it makes possible further application of gene therapy for the treatment of other rare and not amenable to traditional drugs in disease"
The company also announced that it intends to promote their drug for approval in the U.S. and Canada.

The first country in the world that has approved a gene therapy, was China. It happened in 2003, when the company Shenzhen SiBiono GenTech obtained a license of the State administration for food and drug administration of China for the commercial use of recombinant gene therapy Ad-p53 for the treatment of squamous cell carcinoma of the head and neck. This type of cancer accounts for about 10% of the 2.5 million new cancer patients in China.
After five years of clinical tests, Chinese researchers came to very good results. The only side effect of Ad-p53 is a fever, but not all patients. After eight weeks of joint use of radiation and gene therapy 64% of patients with advanced tumors showed complete regression and 32% partial.
The example of China shows that gene therapy could be a real ambrosia in modern medicine. As they say, many researchers in China, the procedure for obtaining a license for medicines is much easier than in Europe and the United States. But since in 2012 "caved in" and Europe, it is quite possible that the dominance of gene therapies is not far off.
Source: /users/104