183
Structural supercapacitors will replace batteries
Is it possible to imagine a future in which electronics will be rid of batteries, cords, sockets, forks and generally from any external power sources? Today, such a prospect seems incredible to ordinary people, but scientists are taking it quite seriously.
Storing electrical energy in a laptop case, electric vehicle chassis or in the walls of a home will make possible a small, nondescript gray plate developed by researchers at Vanderbilt University’s Nanomaterials and Energy Devices Laboratory.
“This device demonstrates, for the first time as far as we can tell, that it is possible to create materials capable of storing and releasing a significant amount of electricity while they are exposed to ordinary static loads and dynamic forces such as vibration or shocks,” says Cary Pint, assistant professor of mechanics.
The new device, developed by graduate student Andrew Westover and Pint, is a supercapacitor that stores energy by collecting charged ions from the surface of a porous material, unlike batteries that use chemical reactions to do so. As a result, supercapacitors can charge and discharge in seconds rather than hours, and keep millions of charge-discharge cycles running instead of thousands like batteries.
In a report on their work published on May 19, 2014 in the journal Nano Letters, Pint and Westover write that their new structural (load-bearing) supercapacitor works flawlessly, storing and discharging charge when exposed to pressures of up to 44 pounds of force per square inch (0.303 MPa), and oscillating accelerations of more than 80 g, significantly more than the turbine blades of a jet engine experience. Mechanical strength does not affect its ability to store and store energy.
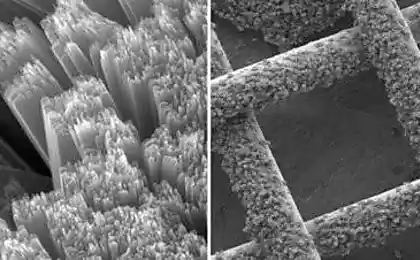
The new supercapacitor looks like a thin gray plate made of silicon electrodes that have been chemically treated so that nanoscale pores form inside them. Outside, the electrodes protect an ultrathin layer of carbon. Between the electrodes is a polymer film that holds the charged ions and plays the same role as the electrolyte in the battery. When compressed, the polymer penetrates the tiny pores of the electrodes, like melted cheese into tightly pressed sandwich loaves.
After cooling and hardening, the polymer becomes extremely durable. The biggest problem in the development of load-bearing supercapacitors Westover calls the prevention of their stratification. But researchers were able to deal with it. “The bonding of a nanoporous material with a polymer electrolyte binds more strongly than superglue,” says the graduate student.
Supercapacitors are significantly behind lithium - ion batteries in specific capacity. In order to operate on the same amount of energy, the capacitor must be much larger and heavier than the battery. But while the supercapacitor stores ten times less energy, it lasts a thousand times longer.
According to the researchers, due to their properties, silicon structural supercapacitors are ideal for use in consumer electronics enclosures and solar panels. However, Pint and Westover believe that the general principles of their construction can be transferred to other materials, such as carbon nanotubes or aluminum.
Source: facepla.net