140
Scientists have discovered an amazing property of gold nanoparticles
Researchers at the Helmholtz Center and Humboldt University have discovered the extraordinary property of gold nanoparticles to assemble into small clusters when dissolved in a special solution.
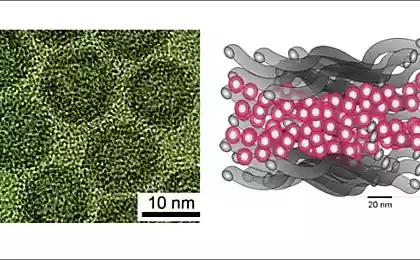
Cryogenic TEM micrograph of gold nanoparticles (Au-NP) in a DES solvent.
Scientists could determine the unique feature of gold nanoparticles using small-angle scattering of X-rays. Subsequent careful examination with an electron microscope confirmed the results. Berlin scientist Armin Hoell says: “We are convinced that such nanoclusters are used as catalysts, in fuel cells, in photocatalytic water splitting, or for other important reactions in chemical engineering.” Professor Klaus Rademann said: What is special about the new process is that it is extremely simple and works with an environmentally friendly and inexpensive solvent.
This solvent consists of two powders: an additive in chicken feed (choline chloride, otherwise vitamin B), and urea. British scientists discovered several years ago that mixing two powders forms a clear liquid capable of dissolving metal oxides, a deep eutectic solvent (DES). German researchers bombarded gold foil with inert gas ions to separate individual atoms.
Scientists assumed that prolonged bombardment will cause the nanoparticles to increase. However, they stopped growing at a size of 5 nanometers. In addition, the microscopic particles did not spread uniformly through the solvent, but began to collect in small groups or clusters. According to the researchers, it is the solvent (DES) that plays a major role in this self-organizing process.
Source: nauka24news.ru/
Cryogenic TEM micrograph of gold nanoparticles (Au-NP) in a DES solvent.
Scientists could determine the unique feature of gold nanoparticles using small-angle scattering of X-rays. Subsequent careful examination with an electron microscope confirmed the results. Berlin scientist Armin Hoell says: “We are convinced that such nanoclusters are used as catalysts, in fuel cells, in photocatalytic water splitting, or for other important reactions in chemical engineering.” Professor Klaus Rademann said: What is special about the new process is that it is extremely simple and works with an environmentally friendly and inexpensive solvent.
This solvent consists of two powders: an additive in chicken feed (choline chloride, otherwise vitamin B), and urea. British scientists discovered several years ago that mixing two powders forms a clear liquid capable of dissolving metal oxides, a deep eutectic solvent (DES). German researchers bombarded gold foil with inert gas ions to separate individual atoms.
Scientists assumed that prolonged bombardment will cause the nanoparticles to increase. However, they stopped growing at a size of 5 nanometers. In addition, the microscopic particles did not spread uniformly through the solvent, but began to collect in small groups or clusters. According to the researchers, it is the solvent (DES) that plays a major role in this self-organizing process.
Source: nauka24news.ru/