1559
Not so scary silver nanoparticles as its ion ...
No doubt: for science a long time ago is no secret that the silver ions formed during surface oxidation, deadly for germs. Silver nanoparticles are applied accurately everywhere: in cosmetic products, socks, containers for storing food supplies, washing powders and sprays, and even where we would like to avoid the uncontrolled proliferation of unwanted microorganisms. However, scientists have suspected that the iron particles and silver alone for themselves have the potential to be toxic to bacteria, individually for the smallest, the value of which is not much more than 3 nm.
So, an ad in a magazine Nano Letters authored by researchers from Rice University (USA) shows that this is not the truth.
In reality, when the possibility of oxidation of the silver is not possible, the nanoparticles do not represent any danger for bacteria. Thus, yielding to the charm of Arcane word "nano" in the large mass of the most various products using a substance, the device acts which have not been fully understood. With silver that could lead to this totally useless supplements (and empty overpayment), and in the case with other new-fangled microparticles with just unknown toxicity and potential influence on the environment in different criteria, the consequences have the potential to be much sadder.
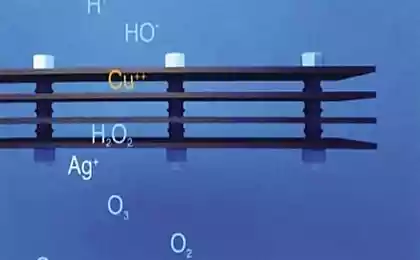
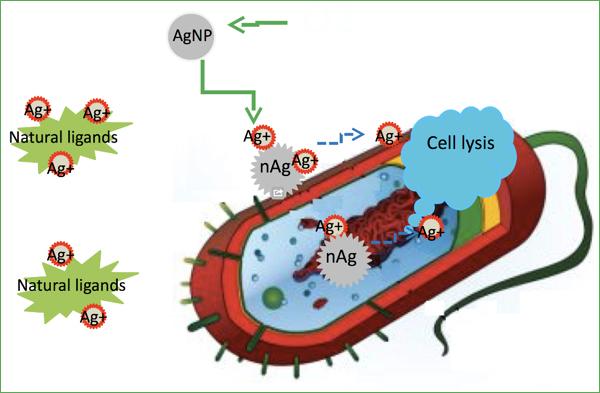
Now we all know that the insoluble silver nanoparticles do not kill cells in direct contact with them. However, after activation by oxidation of soluble ions in some places near the great bacteria are controlled with their killerskimi obligations. To get this simple answer, scientists began with dividing the particle size, because they felt that the toxicity of nanoparticles may inversely proportional to their size. The first results of the test particles 3-11 nm were haphazard ... that, of course, disappointing.
It was then that the thought came to test the toxicity of nanoparticles in an anaerobic environment - in the anoxic chamber to limit the release of silver ions. In such circumstances, all the silver particles were much less toxic to microbes than silver ions. After that, it was decided to hold himself in the synthesis of nanoparticles completely anoxic conditions, that is guaranteed to eliminate even the smallest possibility of oxidation. In this case, the nanoparticles do not exhibit absolutely no toxicity to microbes even at concentrations up to 195 ppm. For comparison, the concentration of silver ions in the 15 parts per billion (!) Is rather, in order to destroy all of the bacteria present in the solution. This means that the toxicity of silver nanoparticles Least least 7665 times lower than the toxicity of its ions. Or, in other words, it is negligible.
Why is this principle? Always be aware that actually describes the same or another property, in order to get a chance to manage it, without falling into the wretched situation. For example, in the case of E. coli silver ions initiate growth of this bacteria to us, if their concentration in solution too low to destroy it. And this is the damage that can cause accident, elementary putting something silver in the water is not providing sufficient number of oxygen under the hood.
So, to silver nanoparticles were truly effective, you need to learn to control the rate of release of ions to achieve their desired concentration.
Experimental "wagon" will compete with several types of cancer
Silk help keep vaccines without costly cooling