493
Nanoparticles of rare metals and light to turn carbon dioxide into fuel
Using only light and tiny nanoparticles of rare metal rhodium, researchers from Duke University have found a way to turn carbon dioxide into a component of fuel. In a recently discovered chemical reaction you can use natural sunlight to reduce the increasing level of CO2 in the atmosphere without the formation of undesirable byproducts, such as toxic carbon monoxide.
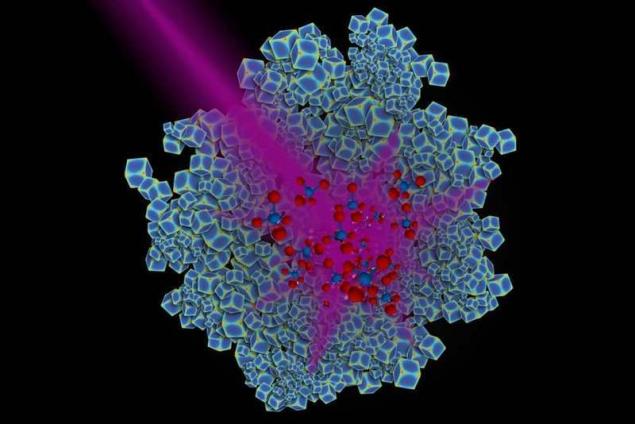
The use of light rather than heat not only more efficiently by the reaction with rhodium, but also contributes to the formation mainly of methane, but not undesirable by-products.
The powdery material composed of nanoparticles of rhodium was placed in a cell reaction with a mixture of carbon dioxide and hydrogen. When nanoparticles were exposed to a powerful UV led, the reaction was held at room temperature with the receipt solely of methane, and when the reaction took place at a temperature of 300 degrees Celsius, the result is a mixture with equal parts of methane and poisonous carbon monoxide.
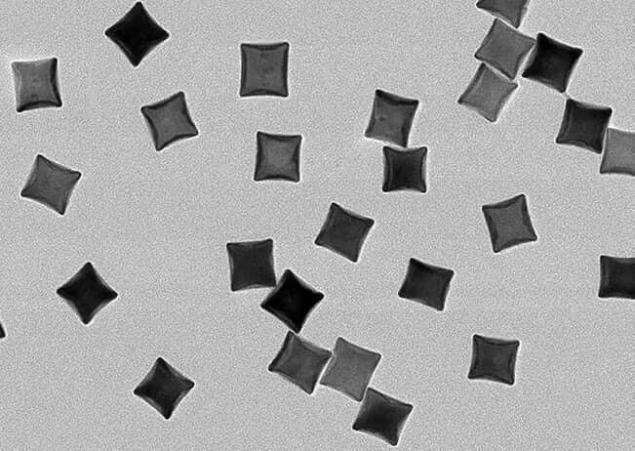
Rhodium in small quantities is used as a catalyst to speed up processes in the manufacture of fertilizers, detergents and pharmaceuticals. It is also used as catalytic Converter in the exhaust systems of cars.
Now the researchers intend to test the efficiency of solar light in other reactions where rhodium is used as catalyst. They also hope to use rhodium to improve the performance of solar cells. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: ecotechnology

The use of light rather than heat not only more efficiently by the reaction with rhodium, but also contributes to the formation mainly of methane, but not undesirable by-products.
The powdery material composed of nanoparticles of rhodium was placed in a cell reaction with a mixture of carbon dioxide and hydrogen. When nanoparticles were exposed to a powerful UV led, the reaction was held at room temperature with the receipt solely of methane, and when the reaction took place at a temperature of 300 degrees Celsius, the result is a mixture with equal parts of methane and poisonous carbon monoxide.

Rhodium in small quantities is used as a catalyst to speed up processes in the manufacture of fertilizers, detergents and pharmaceuticals. It is also used as catalytic Converter in the exhaust systems of cars.
Now the researchers intend to test the efficiency of solar light in other reactions where rhodium is used as catalyst. They also hope to use rhodium to improve the performance of solar cells. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: ecotechnology
Released lamp which 90% will reduce the cost of electricity
Corvette battery powered speed of up to 306 kilometers per hour