623
Simple Science # 6 or experiments at home
Sources: HabraHabr and Simple Science on GTV.
The sixth edition of the weekly fun experiments explaining how they can be done.
Below 6 experiments. Please do not break.
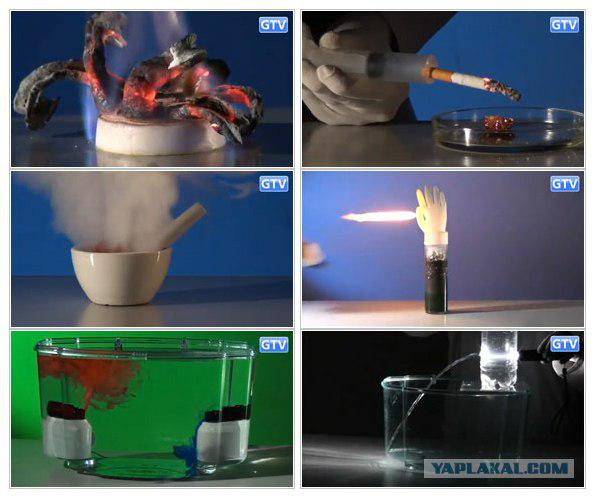
For the experiment, you will need: tablets of calcium gluconate, dry fuel, matches or a gas burner.
1) ignite dry fuel.
2) Put several tablets of calcium gluconate in the burning dry fuel.
Result: the thermal decomposition of the tablets of calcium gluconate is produced, so-called "Pharaoh's snake." Light gray shade the "snake" is obtained by the reaction of calcium oxide.
Posted in [mergetime] 1338981320 [/ mergetime]
For the experiment, you will need: a cigarette, a disposable syringe, lighter, refined sugar.
1) The disposable syringe bore holes 8mm equal to the diameter of the cigarette filter.
2) Insert the cigarette into the syringe and "smoked" it by moving the plunger of the syringe.
3) Take one cube refined and lights it from the burner.
4) The second cube, previously sprinkled with ashes, burned, too.
Bottom line: if you burn the sugar, it will not burn, but only to melt. But if you sprinkle the ashes of his cigarette - he lights up. This is due to the fact that the ash contains lithium salts, which will be a catalyst for the reaction.
Posted in [mergetime] 1338981436 [/ mergetime]
For the experiment, you will need: hot and cold water, the aquarium with room temperature water, blue and red colors, two of the same bubble, two stands.
1) One vial pour cold water and add to it a blue dye.
2) In another vial, pour hot water and add red dye in it.
3) Pour into the aquarium water at room temperature.
4) Put the aquarium two stand on opposite sides.
5) Take two bottles in both hands, fingers plugging holes, omit the aquarium and put them on the stand.
Bottom line: as we know, tends to warm up and cool down. In this test, due to the added colorants, this effect is clearly demonstrated in the liquid: hot (red), the water rises to the surface, whereas the cold (blue) tends towards the bottom.
For the experiment, you will need: spacious bulb, water, copper sulphate, sodium chloride, aluminum foil, a gas burner, two vessels for solutions.
1) The one-pot making copper sulfate solution with water.
2) In the second, a solution of salt and water in the same proportion as the bluestone.
3) Pour the contents of the two vessels in a capacious flask.
4) is made from food aluminum foil several lumps in diameter with a flask and put them in the resulting solution in the flask.
5) should be fitted to the top of the bulb rubber glove.
Bottom line: if you mix copper sulphate and sodium chloride with water, then add to the aluminum (You can use a food aluminum foil), highlight hydrogen.
Be careful, because hydrogen is very flammable material! Take this experience away from open flame. If you are going to set fire to the hydrogen produced, then this should be done in a special room.
Posted in [mergetime] 1338981787 [/ mergetime]
For the experiment, you will need: a plastic bottle, a flashlight.
1) The plastic bottle caves below the middle.
2) We fill the bottle with water and screw cap.
3) directs the beam from a flashlight through the bottle so that it would just go out of the hole.
4) screw cap from the bottle.
The result: a beam of light will follow the stream of water, even if it is bent. This effect is used in fiber-optic cables for data transmission over long distances.
Posted in [mergetime] 1338981898 [/ mergetime]
For the experiment, you will need: gidroperita pills, tablets Analgin cup for mixing.
1) Put the tablet in a cup and gidroperita crush.
2) Add in a cup 2 tablet dipyrone and also pulverized.
3) Wait a few minutes.
Bottom line: the interaction with gidroperita analginum stands acrid white smoke. The reaction proceeds in 3-4 minutes at room temperature. However, if the ambient temperature increase (put on the battery, for example), then the reaction time can be shortened.
This experience should be prodelyvat in a well ventilated area.
That's all! Enjoy watching!
Source:
The sixth edition of the weekly fun experiments explaining how they can be done.
Below 6 experiments. Please do not break.

For the experiment, you will need: tablets of calcium gluconate, dry fuel, matches or a gas burner.
1) ignite dry fuel.
2) Put several tablets of calcium gluconate in the burning dry fuel.
Result: the thermal decomposition of the tablets of calcium gluconate is produced, so-called "Pharaoh's snake." Light gray shade the "snake" is obtained by the reaction of calcium oxide.
Posted in [mergetime] 1338981320 [/ mergetime]
For the experiment, you will need: a cigarette, a disposable syringe, lighter, refined sugar.
1) The disposable syringe bore holes 8mm equal to the diameter of the cigarette filter.
2) Insert the cigarette into the syringe and "smoked" it by moving the plunger of the syringe.
3) Take one cube refined and lights it from the burner.
4) The second cube, previously sprinkled with ashes, burned, too.
Bottom line: if you burn the sugar, it will not burn, but only to melt. But if you sprinkle the ashes of his cigarette - he lights up. This is due to the fact that the ash contains lithium salts, which will be a catalyst for the reaction.
Posted in [mergetime] 1338981436 [/ mergetime]
For the experiment, you will need: hot and cold water, the aquarium with room temperature water, blue and red colors, two of the same bubble, two stands.
1) One vial pour cold water and add to it a blue dye.
2) In another vial, pour hot water and add red dye in it.
3) Pour into the aquarium water at room temperature.
4) Put the aquarium two stand on opposite sides.
5) Take two bottles in both hands, fingers plugging holes, omit the aquarium and put them on the stand.
Bottom line: as we know, tends to warm up and cool down. In this test, due to the added colorants, this effect is clearly demonstrated in the liquid: hot (red), the water rises to the surface, whereas the cold (blue) tends towards the bottom.
For the experiment, you will need: spacious bulb, water, copper sulphate, sodium chloride, aluminum foil, a gas burner, two vessels for solutions.
1) The one-pot making copper sulfate solution with water.
2) In the second, a solution of salt and water in the same proportion as the bluestone.
3) Pour the contents of the two vessels in a capacious flask.
4) is made from food aluminum foil several lumps in diameter with a flask and put them in the resulting solution in the flask.
5) should be fitted to the top of the bulb rubber glove.
Bottom line: if you mix copper sulphate and sodium chloride with water, then add to the aluminum (You can use a food aluminum foil), highlight hydrogen.
Be careful, because hydrogen is very flammable material! Take this experience away from open flame. If you are going to set fire to the hydrogen produced, then this should be done in a special room.
Posted in [mergetime] 1338981787 [/ mergetime]
For the experiment, you will need: a plastic bottle, a flashlight.
1) The plastic bottle caves below the middle.
2) We fill the bottle with water and screw cap.
3) directs the beam from a flashlight through the bottle so that it would just go out of the hole.
4) screw cap from the bottle.
The result: a beam of light will follow the stream of water, even if it is bent. This effect is used in fiber-optic cables for data transmission over long distances.
Posted in [mergetime] 1338981898 [/ mergetime]
For the experiment, you will need: gidroperita pills, tablets Analgin cup for mixing.
1) Put the tablet in a cup and gidroperita crush.
2) Add in a cup 2 tablet dipyrone and also pulverized.
3) Wait a few minutes.
Bottom line: the interaction with gidroperita analginum stands acrid white smoke. The reaction proceeds in 3-4 minutes at room temperature. However, if the ambient temperature increase (put on the battery, for example), then the reaction time can be shortened.
This experience should be prodelyvat in a well ventilated area.
That's all! Enjoy watching!
Source: