500
The Nobel prize in chemistry was given for the fluorescence microscopy, high-resolution
Sixty two million three hundred eighty four thousand five hundred ninety four
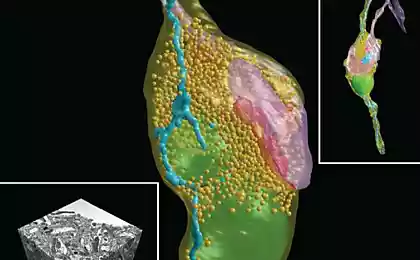
The work of Stefan Halle, Eric Betzig and William Marner allowed to see in a cage separate molecules.
To examine the cage and its contents, we must take a microscope. Its working principle is relatively simple: light rays pass through the object, and then fall into a magnifying lens, so that we can see the cell and some organelles within it, for example, the nucleus or the mitochondria.
But if we want to see a protein molecule, or DNA, or to consider a large supramolecular complex like the ribosome or viral particle, an ordinary light microscope will be useless. In 1873, German physicist Ernst Abbe derived the formula, consider the limit of any optical microscope: it turns out, you cannot see object smaller than half the wavelength of visible light – i.e. less than 0.2 micrometers.
Sixty eight million six hundred eighty seven thousand seven hundred fifty three
The solution, obviously, is to choose something that would be able to replace the visible light. You can use a beam of electrons, and then we get electron microscope it is possible to observe viruses and protein molecules, but the observed objects by electron microscopy fall into a completely unnatural conditions. So very successful was the idea of Hella Stefan (Stefan W. Hell) from the Institute for biophysical chemistry, max Planck Society (Germany), who in the early 90-ies came up with the idea to use for visualization of macromolecules and their complexes stimulated fluorescent radiation.
Twenty eight million six hundred eighty four thousand three hundred seventy
The idea was that the object can be irradiated with a laser beam, which translates biological molecules in the excited state. From this state they start to change to normal to get rid of excess energy in the form of light – that is, there are fluorescence, and the molecule will become visible. But the radiated waves will be of various lengths, and in front of us is uncertain spot. To avoid this from happening, along with the stimulating laser, the object is processed by the quenching beam, which suppresses all the waves except those which have a nanometer length. Radiation with a wavelength of the order of nanometers allows to distinguish one molecule from another.
Thirty three million twenty six thousand five hundred thirty nine
Method is called STED (stimulated emission depletion), and just behind him, Stefan hell received his award from the Nobel prize. In STED-microscopy the object is not covered by laser excitation at once, as it draws two thin beams of light (exciter and damper), because the smaller the area that is fluorescing at a given time, the higher the image resolution.
Eighty five million two hundred twenty seven thousand forty five
STED method was later supplemented by the so-called dnamolecules microscopy, developed in the late twentieth century independently by two other current winners, Eric Bettega (Eric Betzig) from the Institute of Howard Hughes and William Merner (William E. Moerner) from Stanford. In most physico-chemical methods relying on fluorescence, we observe the total radiation from many molecules. William Marner was suggested by the method by which it is possible to observe the emission of a single molecule. Experimenting with green fluorescent protein (GFP), he noticed that his molecules glow can be arbitrarily turned on and off by manipulating the length of the exciting wave. Turning on and off the fluorescence of different GFP molecules, they can be observed in the light microscope, not paying attention to the nanometer limit of Abbe. The whole image could be obtained simply by combining several shots with different luminous molecules in the surveillance field. These data were supplemented by the ideas of Eric Betzig, who proposed to increase the resolution of fluorescence microscopy using proteins with different optical properties (that is, roughly speaking, mixed).
Thirty six million three hundred nine thousand eight hundred seventy one
The combination of the method of excitation-damping Hella with the method the amount of overlap of Betzig and Marner allowed to develop microscopy with nanometer resolution. With its help we can not only observe organelles and their fragments, but also of the interaction of molecules with each other (if the fluorescent molecules to tag proteins), which, again, is not always possible with electron-microscopic methods. The value of the method is difficult to overestimate, because of intermolecular contacts is what is on molecular biology, and without which it is impossible, for instance, or the creation of new medicines, nor the deciphering of genetic mechanisms, nor many other things that lie in the field of modern science and technology.
Prepared according to the Nobel Committee.
The work of Stefan Halle, Eric Betzig and William Marner allowed to see in a cage separate molecules.
To examine the cage and its contents, we must take a microscope. Its working principle is relatively simple: light rays pass through the object, and then fall into a magnifying lens, so that we can see the cell and some organelles within it, for example, the nucleus or the mitochondria.
But if we want to see a protein molecule, or DNA, or to consider a large supramolecular complex like the ribosome or viral particle, an ordinary light microscope will be useless. In 1873, German physicist Ernst Abbe derived the formula, consider the limit of any optical microscope: it turns out, you cannot see object smaller than half the wavelength of visible light – i.e. less than 0.2 micrometers.
Sixty eight million six hundred eighty seven thousand seven hundred fifty three
The solution, obviously, is to choose something that would be able to replace the visible light. You can use a beam of electrons, and then we get electron microscope it is possible to observe viruses and protein molecules, but the observed objects by electron microscopy fall into a completely unnatural conditions. So very successful was the idea of Hella Stefan (Stefan W. Hell) from the Institute for biophysical chemistry, max Planck Society (Germany), who in the early 90-ies came up with the idea to use for visualization of macromolecules and their complexes stimulated fluorescent radiation.
Twenty eight million six hundred eighty four thousand three hundred seventy
The idea was that the object can be irradiated with a laser beam, which translates biological molecules in the excited state. From this state they start to change to normal to get rid of excess energy in the form of light – that is, there are fluorescence, and the molecule will become visible. But the radiated waves will be of various lengths, and in front of us is uncertain spot. To avoid this from happening, along with the stimulating laser, the object is processed by the quenching beam, which suppresses all the waves except those which have a nanometer length. Radiation with a wavelength of the order of nanometers allows to distinguish one molecule from another.
Thirty three million twenty six thousand five hundred thirty nine
Method is called STED (stimulated emission depletion), and just behind him, Stefan hell received his award from the Nobel prize. In STED-microscopy the object is not covered by laser excitation at once, as it draws two thin beams of light (exciter and damper), because the smaller the area that is fluorescing at a given time, the higher the image resolution.
Eighty five million two hundred twenty seven thousand forty five
STED method was later supplemented by the so-called dnamolecules microscopy, developed in the late twentieth century independently by two other current winners, Eric Bettega (Eric Betzig) from the Institute of Howard Hughes and William Merner (William E. Moerner) from Stanford. In most physico-chemical methods relying on fluorescence, we observe the total radiation from many molecules. William Marner was suggested by the method by which it is possible to observe the emission of a single molecule. Experimenting with green fluorescent protein (GFP), he noticed that his molecules glow can be arbitrarily turned on and off by manipulating the length of the exciting wave. Turning on and off the fluorescence of different GFP molecules, they can be observed in the light microscope, not paying attention to the nanometer limit of Abbe. The whole image could be obtained simply by combining several shots with different luminous molecules in the surveillance field. These data were supplemented by the ideas of Eric Betzig, who proposed to increase the resolution of fluorescence microscopy using proteins with different optical properties (that is, roughly speaking, mixed).
Thirty six million three hundred nine thousand eight hundred seventy one
The combination of the method of excitation-damping Hella with the method the amount of overlap of Betzig and Marner allowed to develop microscopy with nanometer resolution. With its help we can not only observe organelles and their fragments, but also of the interaction of molecules with each other (if the fluorescent molecules to tag proteins), which, again, is not always possible with electron-microscopic methods. The value of the method is difficult to overestimate, because of intermolecular contacts is what is on molecular biology, and without which it is impossible, for instance, or the creation of new medicines, nor the deciphering of genetic mechanisms, nor many other things that lie in the field of modern science and technology.
Prepared according to the Nobel Committee.