404
The most efficient thermoelectric supercapacitor can be charged-even from the sun's heat
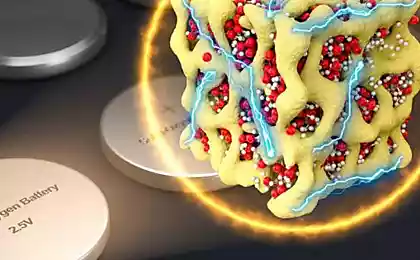
In the laboratory of organic electronics Germany University (Sweden) have developed a supercapacitor that overharvesting converts heat into electricity. It can be recharged from the Sun or collect heat energy from other sources, for example from some production process.
Supercapacitor does not contain expensive or hazardous materials in any significant quantities and is completely suitable for production on an industrial scale in existing production lines. The inventors hope that a new type of thermoelectric devices will be widely used, so we'll no longer aimlessly dissipate heat into the atmosphere.
A supercapacitor is a capacitor with organic or inorganic electrolyte, electrodes which serves as an electric double layer at the interface of electrolyte and electrode. The thickness of the electrical double layer is extremely small, and the area of the porous material of the plates is very large, so that the stored energy is higher compared to conventional capacitors.
When you create a new supercapacitor, the researchers used a thermodynamic effect (the effect of the litter) — the tension between contacts with different temperatures.
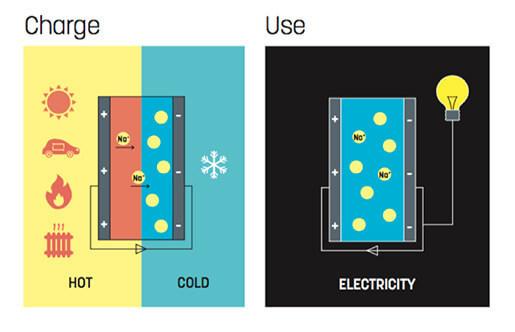
Many years in the laboratory experimenting with different types of electrolytes are ions, and conductive polymers. Positive ions are small and quick, and the negatively charged polymer is relatively bulky and heavy. In the case of a temperature difference between the cations move to the cold electrode quickly, and the anions remain in place. The charge accumulated in the layer of carbon nanotubes.
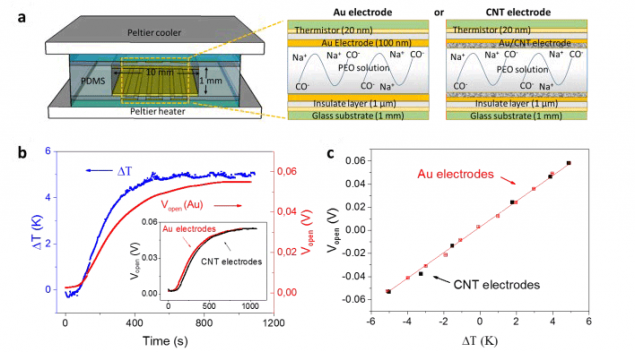
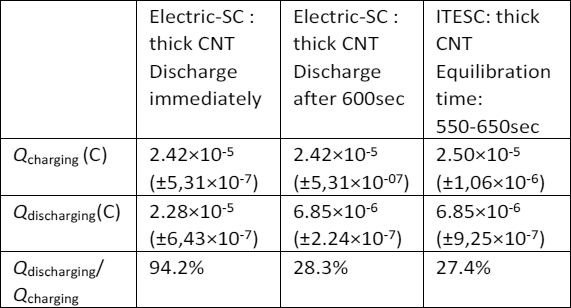
The researchers were able to pick up the electrolyte is thousands of times better than any existing supercapacitors on thermoelectric effect. The electrolyte is made on the basis of polyethylene glycol (PEO) with the addition of NaOH (C(NaOH)=0.75 mM). One electrode of the supercapacitor are made of gold, the second carbon nanotubes.
The inventors recognized that they themselves do not understand why their thermoelectric supercapacitor is 2500 times more effective than all existing ones.
published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/272658/
Supercapacitor does not contain expensive or hazardous materials in any significant quantities and is completely suitable for production on an industrial scale in existing production lines. The inventors hope that a new type of thermoelectric devices will be widely used, so we'll no longer aimlessly dissipate heat into the atmosphere.
A supercapacitor is a capacitor with organic or inorganic electrolyte, electrodes which serves as an electric double layer at the interface of electrolyte and electrode. The thickness of the electrical double layer is extremely small, and the area of the porous material of the plates is very large, so that the stored energy is higher compared to conventional capacitors.
When you create a new supercapacitor, the researchers used a thermodynamic effect (the effect of the litter) — the tension between contacts with different temperatures.

Many years in the laboratory experimenting with different types of electrolytes are ions, and conductive polymers. Positive ions are small and quick, and the negatively charged polymer is relatively bulky and heavy. In the case of a temperature difference between the cations move to the cold electrode quickly, and the anions remain in place. The charge accumulated in the layer of carbon nanotubes.

The researchers were able to pick up the electrolyte is thousands of times better than any existing supercapacitors on thermoelectric effect. The electrolyte is made on the basis of polyethylene glycol (PEO) with the addition of NaOH (C(NaOH)=0.75 mM). One electrode of the supercapacitor are made of gold, the second carbon nanotubes.
The inventors recognized that they themselves do not understand why their thermoelectric supercapacitor is 2500 times more effective than all existing ones.

published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/272658/