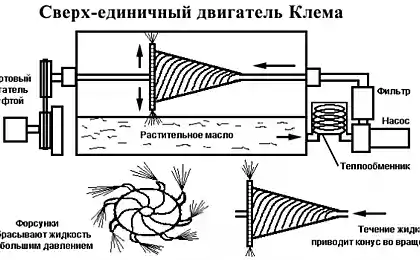
726
Death from classical thermodynamics
Perpetual motion machine of the second kind, perhaps, save the Earth from the "heat death»
The speed with which humanity turns into heat all other forms of energy is already beginning to threaten the very existence of civilization. "Heat Death" in the foreseeable future because of all the growing energy consumption, followed by its scattering in the form of heat already seems inevitable if the current pace of economic development. But if humanity will try to slow them down, then go across the laws of evolution and still be lost.
Is there a way out? It is possible that he has not yet seen simply because of the misunderstanding of the physical principle. Conversion of energy consumption in the energy cycle in principle would allow to increase the intensity, without disturbing the medium. This proves that the experience of the organic world, which for thousands of years while maintaining the mass of the biosphere more or less constant, repeatedly increased its evolution during the annual consumption of energy and matter. Now skip them annually through an array of material comparable to the mass of the Earth's crust, and by some estimates - higher than it.
Perpetual motion machine of the second kind is impossible?
Since almost all our consumption of energy eventually dissipates as heat, because of what threatens us "heat death", insofar cycling power will have to take the form of heat cycle. In other words, we have to learn how to assemble the scattered heat again and again to use his energy.
Ideal heat engine is considered to be that which is theoretically designed in 1824 by French physicist Sadi Carnot (Nicolas Léonard Sadi Carnot, 1796-1832). Its perfect is that the coefficient of performance (COP) of any other machine that uses the same refrigerator and heater will be less than that of the machine, invented them.
Collect the heat dispersed in the environment, it can be a variety of ways and for different purposes. Quite a popular way of home heating - heat pump. It works on the principle opposite to the refrigeration system and produces heat with minimal cost of other forms of energy (eg electricity). Photos (Creative Commons license): Ryan Somma

The fact that the efficiency of his machine is not unity, it follows from the fact of it having a refrigerator having received a certain energy from the heater (such as heat of combustion), the working fluid (in the ideal machine is, of course, ideal gas) doing useful work is completely useless a portion of their energy as heat cooler. The Carnot engine as the working fluid using an ideal gas (ie its volume when the temperature is lowered can be made arbitrarily small), the cylinder must be thermally insulating, and each process cycle - or isothermal (ie, go at a constant temperature) or adiabatic (ie going to the exclusion of the possibility of the exchange of energy with the environment.
Today, for the gathering of scattered power plants use the heat of the classical type (with a fridge) - geo- and hydrothermal power plants and heat pumps with efficiency lower than the Carnot efficiency.
Of course, the use of ambient heat is possible only because that the medium is heated unevenly, ie with changes in temperature, which used thermal heat collecting machines. As soon as the magnitude of these differences is small, the efficiency of classical heat engines stabbed to extremely low values. Therefore, the heat cycle in the energy sector can become real only when it is basing on power plants without a refrigerator, the efficiency of which would not be limited to the Carnot efficiency.
These power plants are called perpetual motion machines of the second kind. It is believed that they are forbidden second law of thermodynamics. However, the threat of a "heat death" makes us the most sympathetic consideration to the arguments in their defense.
The situation is not hopeless. It can not be so over millions and billions of years, the laws of evolution buoyed the organic world, and then the mankind to develop in a certain direction (towards intensification of consumption of matter and energy), and then this development suddenly stumbled to a law of physics that, making it impossible to heat cycle, mankind would be doomed to failure. The laws of evolution and physics, I think, are part of a single and consistent set of laws of nature. If this is indeed the case, the ban on the perpetual motion of the second kind must be insolvent.
French physicist Sadi Carnot created his theory of heat engines, when he was still very young. Although the basis of his reasoning lay subsequently rejected the theory of the indestructibility of the thermal fluid, many of its findings were accurate and had great practical benefit

Errors classics
Of course, science does not happen without mistakes, but in the history of the prohibition of perpetual motion of the second kind of error particularly large. Start with the fact that the withdrawal of the mandatory Sadi Carnot refrigerator was made on the basis of the principle of the indestructibility of caloric, according to which the consumption of heat like energy consumption. By consuming energy, we do not destroy it (as the law of conservation of energy), but only one convert it to another form. Caloric intake, said Carnot, does not mean its destruction, but it is only a transition from a warm body to a less warm. That's less than a warm body and is believed Carnot, a refrigerator, a must for any heat engine:
The appearance of the driving force is obliged to steam engines do not actually spend caloric, and its transition from the hot to the cold body [...] This principle applies to all cars, driven by the heat of [...] According to this principle, not enough to create heat to cause a driving force : you still get cold.
Discarding the theory of caloric, marching for classical thermodynamics Carnot upheld his conclusion about the presence of any heat engine cooler. To put it mildly, this is surprising, since this is well known that, turning into other forms of energy, heat ceases to exist as heat. In other words, consuming caloric (heat), we destroy that undermines the argument Carnot.
Even more amazing History Of perpetual motion machine of the second kind. It was introduced by Wilhelm Ostwald (Wilhelm Friedrich Ostwald, 1853-1932) in 1888, and he did it absolutely incorrect:
Jobs, delivered a giant ocean liner machine entirely converted into heat as the energy of the moving ship on arrival becomes zero and turns into heat. If one had a message seawater heat back to transform into kinetic energy, the ship could make his way back without the cost of coal, which, of course, can not be [...] Implementation of this would be tantamount perpetuum mobile [...] since the same the amount of energy would be constantly consumed for the same transitions, the gratuitous technical problem getting a job would be considered permissible. What this in fact is not, expressed in the following form: perpetuum mobile of the second kind is impossible. Thus, under the perpetuum mobile of the second kind imply a machine that can produce energy at rest or in motion to transform it into other forms.
Resting energy Ostwald, as it was then assumed names scattered among warm:
Gifts of warmth is everywhere in the infinite number of [...] it is [a] resting energy.
Please note: Ostwald prohibits not heat engine without refrigeration, but any heat engine, which consumes heat dissipation. However, we are with you now know for sure that there are heat engines! Ostwald, suppose he could about them and do not know (the first electricity generated geothermal installation appeared in 1904, a similar hydrothermal - in 1929, the first patent for heat pump technology was issued in 1912), but can not surprise that its wording played during the XX century and the other authors. Action on the world tendency to scattering of non-thermal forms of energy as heat all of them, since Ostwald correctly transformed into the law knows no exceptions.
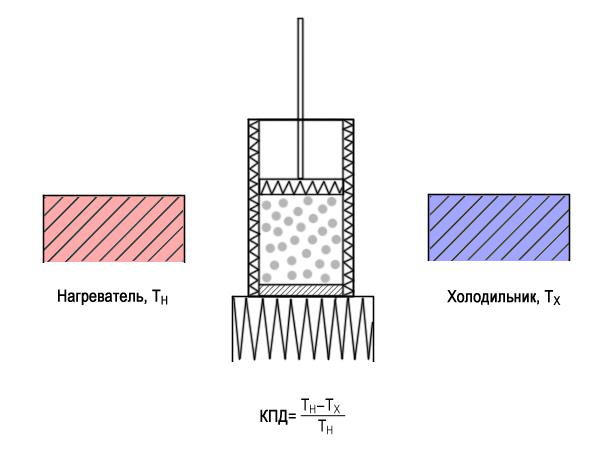
Carnot cycle requires a fairly peculiar device: the cylinder and the piston are made of a perfect thermal insulator, but at the bottom of the cylinder can pass the heat. His alternately put on the heater, the fridge, then heat insulator. To ensure the equilibrium of the piston to move with an infinitely small velocity. Efficiency of any heat engine under the same heater and the refrigerator is less than the calculated value of Carnot. Illustration Oleg Sendyurev / "Around the World»
Refrigerator mandatory?
But back to the ban on thermal machines without refrigeration. Followers Carnot, abandoning caloric does not correct his mistake, in my opinion, because they worked exclusively with classical thermal machines with two features that make the fridge for them and indeed necessary: 1) cyclical; 2) a single-phase working fluid (gas or liquid). Returning a working fluid in the initial state, we have to give part of the heat received from the heater refrigerator. Meanwhile, the non-cyclic heat engines it is not necessary as it is not necessary, presumably, and cyclic heat engines with two-phase working fluid gas-liquid interface.
Examples of non-cyclic heat engine without a refrigerator can serve as working in a vacuum rocket engine for which talk about the cooling of the combustion products behind it is not necessary - the expansion of the gas in the void, as it is known, takes place isothermally. Another example of non-cyclic heat engine without a refrigerator is shown below.
As for the cyclic heat engines with two-phase working fluid, the way to prove it in the last decade independently several local researchers, the return of the working fluid in the initial state can they be carried out not with the transfer of the heat refrigerator, but on its return to the heater. Specifically, part of the useful work of one phase working fluid used for adiabatic expansion and hence cooling of the other. External refrigerator is unnecessary, and efficiency - not limited to the Carnot efficiency of the machine.
The second law of thermodynamics
A favorite argument of defenders of perpetual motion machine of the second kind - the limitations of the second law of thermodynamics. My position is different. I believe that the second - it is the law of nature acting, if we exclude the microscopic fluctuations, without restrictions. Another thing that should be properly read it.
In modern textbooks and academic monographs content of the second start too blurry, as evidenced by the diversity of its wording. I collect them for many years, and so far in my collection of 48 language, but in reality more of them. This diversity contrasts, for example, with the wording of the law of conservation of energy, which are in different sources almost word-for-word repeat each other.
We often read about the identity of the different formulations of the second law. This is both true and not. Of course, all of the many formulations of the second law can not be identical, but they can all be different. I tried vyshelushit core of the law of nature, which is behind all this, and I got two of these components: 1) the law of the increase of the total entropy; 2) there is an asymmetry between transformations nonthermal forms of energy into heat, on the one hand, and the conversion of heat into other forms of energy - the other: first, in contrast to the second, do not require compensation.
The most common source of confusion I see in the inability to distinguish between thermal and non-thermal entropy. Meanwhile, the difference between them is easy to demonstrate. If you mix the hot air with cold, we get warm air - the same thing that the transfer of a certain amount of heat from the hot air cold. Entropy has increased with the participation of the thermal process.
If the mix oxygen with hydrogen at the same temperature, the growth of entropy is not associated with the transfer of heat. When mixing cold with hot oxygen-hydrogen entropy is growing and thanks to equalize the temperature, and due to the simple mixing (diffusion). The problem is that under certain conditions the growth of the total entropy can be accompanied by reduction of one of the terms - for example, heat.
In general, the law increases the total entropy does not apply and the "law" of increasing the thermal entropy. Since thus the thermal entropy can decrease, so far as the conversion of heat into other forms of energy can occur with a decrease in thermal entropy, only to grow full. This means that the conversion of heat into other forms of energy can be full, i.e. to occur without the thermal compensation.
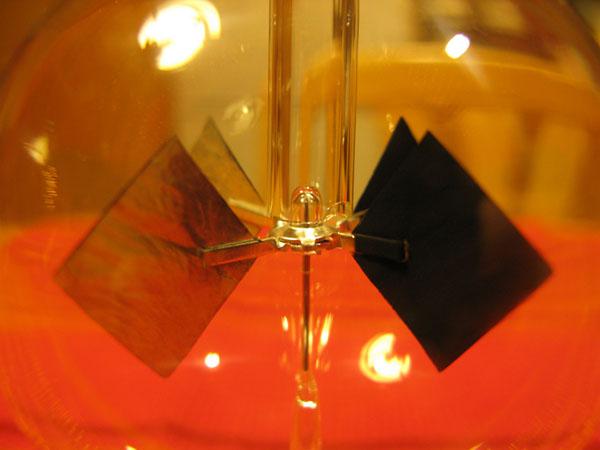
Crookes radiometer shows how thermal energy can be converted into mechanical in nonequilibrium systems. The bright and dark surfaces of the blades are heated by light incident upon them and, in turn, a different heated air in their vicinity. Where the temperature is higher and larger pressure - as a result of the blades begin to rotate. Photos (Creative Commons license): Leo-setä
Obligation of the refrigerator for any heat engine today to explain the need for increasing the thermal entropy. Cancel "law" does it increase the ban on perpetual motion machines of the second kind insolvent, paving the way for the creation of energy, built on the circulation of heat.
On the projects of perpetual motion machine of the second kind
Today, there are dozens of such projects. However, all of them indiscriminately and declared a priori contradictory second law of thermodynamics and therefore unworthy of critical analysis. As a result, the authors are forced to "cook" in your own environment, which, of course, prevent them from becoming the object of rational criticism and reduces the level of scientific texts, often - to unacceptably low. It is very difficult to separate the chaff from the rye. I'll tell only one such project, the idea of which seems to me quite worthy of discussion.
Put into the wind in the atmosphere of a tapered tube where the air is accelerated by "geometric" reasons, like the air in a crevice between rocks or in a narrow passage between the houses. Such a flow of an ideal gas approximation is described by Bernoulli's equation, known in two main forms. The first is that the acceleration of the gas along the line of current is accompanied by a decrease in its pressure, according to the second - a drop in temperature. The first effect of the wing provides the lift, the second, I think, can be laid in the foundation of a perpetual motion machine of the second kind.
In fact, the cooling gas flow means reducing the number of the contained heat acceleration - increase its kinetic energy. The thermal energy is directly converted into kinetic energy here, there is no refrigerator. The cooling gas flow takes place with decreasing thermal entropy, which is compensated by the growth of non-thermal entropy associated with a decrease in pressure.
Narrows the tube may be provided with a turbine, turning it into a power plant. In "Wind Turbine" this kind of Russian inventors received a patent Mikhail Yegorov, Igor Orlov and Emmanuel Avraamovich Sable. Their installation looks in the drawings as a pot-bellied bomb hanging along the airflow and accept it inward annular hole.
The reader has the necessary experimental base (What is missing from the author), he can put the experimentum crucis, using, for example, for the construction of a tapered tube film for greenhouses, secured with steel wire frame.
Installing Egorova-Orlova-Sable, it seems to me, can be adapted to the aquatic environment where it can have a greater capacity per unit volume as the waters of the earth contains much more heat than the atmosphere per unit volume.
But it is not in fact whether this particular design. My tasks included the presentation of projects is not perpetual motion machine of the second kind, which could be run immediately into production. I'm just trying to reverse a steady negative attitude of Big Science to the very idea of such engines.
Sergei Haytun,
www.vokrugsveta.ru/telegraph/theory/1185
Fan Dyson works without blades. It looks strange, but there is no contradiction to the laws of physics! Photo: Dyson

Source:
The speed with which humanity turns into heat all other forms of energy is already beginning to threaten the very existence of civilization. "Heat Death" in the foreseeable future because of all the growing energy consumption, followed by its scattering in the form of heat already seems inevitable if the current pace of economic development. But if humanity will try to slow them down, then go across the laws of evolution and still be lost.
Is there a way out? It is possible that he has not yet seen simply because of the misunderstanding of the physical principle. Conversion of energy consumption in the energy cycle in principle would allow to increase the intensity, without disturbing the medium. This proves that the experience of the organic world, which for thousands of years while maintaining the mass of the biosphere more or less constant, repeatedly increased its evolution during the annual consumption of energy and matter. Now skip them annually through an array of material comparable to the mass of the Earth's crust, and by some estimates - higher than it.
Perpetual motion machine of the second kind is impossible?
Since almost all our consumption of energy eventually dissipates as heat, because of what threatens us "heat death", insofar cycling power will have to take the form of heat cycle. In other words, we have to learn how to assemble the scattered heat again and again to use his energy.
Ideal heat engine is considered to be that which is theoretically designed in 1824 by French physicist Sadi Carnot (Nicolas Léonard Sadi Carnot, 1796-1832). Its perfect is that the coefficient of performance (COP) of any other machine that uses the same refrigerator and heater will be less than that of the machine, invented them.
Collect the heat dispersed in the environment, it can be a variety of ways and for different purposes. Quite a popular way of home heating - heat pump. It works on the principle opposite to the refrigeration system and produces heat with minimal cost of other forms of energy (eg electricity). Photos (Creative Commons license): Ryan Somma

The fact that the efficiency of his machine is not unity, it follows from the fact of it having a refrigerator having received a certain energy from the heater (such as heat of combustion), the working fluid (in the ideal machine is, of course, ideal gas) doing useful work is completely useless a portion of their energy as heat cooler. The Carnot engine as the working fluid using an ideal gas (ie its volume when the temperature is lowered can be made arbitrarily small), the cylinder must be thermally insulating, and each process cycle - or isothermal (ie, go at a constant temperature) or adiabatic (ie going to the exclusion of the possibility of the exchange of energy with the environment.
Today, for the gathering of scattered power plants use the heat of the classical type (with a fridge) - geo- and hydrothermal power plants and heat pumps with efficiency lower than the Carnot efficiency.
Of course, the use of ambient heat is possible only because that the medium is heated unevenly, ie with changes in temperature, which used thermal heat collecting machines. As soon as the magnitude of these differences is small, the efficiency of classical heat engines stabbed to extremely low values. Therefore, the heat cycle in the energy sector can become real only when it is basing on power plants without a refrigerator, the efficiency of which would not be limited to the Carnot efficiency.
These power plants are called perpetual motion machines of the second kind. It is believed that they are forbidden second law of thermodynamics. However, the threat of a "heat death" makes us the most sympathetic consideration to the arguments in their defense.
The situation is not hopeless. It can not be so over millions and billions of years, the laws of evolution buoyed the organic world, and then the mankind to develop in a certain direction (towards intensification of consumption of matter and energy), and then this development suddenly stumbled to a law of physics that, making it impossible to heat cycle, mankind would be doomed to failure. The laws of evolution and physics, I think, are part of a single and consistent set of laws of nature. If this is indeed the case, the ban on the perpetual motion of the second kind must be insolvent.
French physicist Sadi Carnot created his theory of heat engines, when he was still very young. Although the basis of his reasoning lay subsequently rejected the theory of the indestructibility of the thermal fluid, many of its findings were accurate and had great practical benefit

Errors classics
Of course, science does not happen without mistakes, but in the history of the prohibition of perpetual motion of the second kind of error particularly large. Start with the fact that the withdrawal of the mandatory Sadi Carnot refrigerator was made on the basis of the principle of the indestructibility of caloric, according to which the consumption of heat like energy consumption. By consuming energy, we do not destroy it (as the law of conservation of energy), but only one convert it to another form. Caloric intake, said Carnot, does not mean its destruction, but it is only a transition from a warm body to a less warm. That's less than a warm body and is believed Carnot, a refrigerator, a must for any heat engine:
The appearance of the driving force is obliged to steam engines do not actually spend caloric, and its transition from the hot to the cold body [...] This principle applies to all cars, driven by the heat of [...] According to this principle, not enough to create heat to cause a driving force : you still get cold.
Discarding the theory of caloric, marching for classical thermodynamics Carnot upheld his conclusion about the presence of any heat engine cooler. To put it mildly, this is surprising, since this is well known that, turning into other forms of energy, heat ceases to exist as heat. In other words, consuming caloric (heat), we destroy that undermines the argument Carnot.
Even more amazing History Of perpetual motion machine of the second kind. It was introduced by Wilhelm Ostwald (Wilhelm Friedrich Ostwald, 1853-1932) in 1888, and he did it absolutely incorrect:
Jobs, delivered a giant ocean liner machine entirely converted into heat as the energy of the moving ship on arrival becomes zero and turns into heat. If one had a message seawater heat back to transform into kinetic energy, the ship could make his way back without the cost of coal, which, of course, can not be [...] Implementation of this would be tantamount perpetuum mobile [...] since the same the amount of energy would be constantly consumed for the same transitions, the gratuitous technical problem getting a job would be considered permissible. What this in fact is not, expressed in the following form: perpetuum mobile of the second kind is impossible. Thus, under the perpetuum mobile of the second kind imply a machine that can produce energy at rest or in motion to transform it into other forms.
Resting energy Ostwald, as it was then assumed names scattered among warm:
Gifts of warmth is everywhere in the infinite number of [...] it is [a] resting energy.
Please note: Ostwald prohibits not heat engine without refrigeration, but any heat engine, which consumes heat dissipation. However, we are with you now know for sure that there are heat engines! Ostwald, suppose he could about them and do not know (the first electricity generated geothermal installation appeared in 1904, a similar hydrothermal - in 1929, the first patent for heat pump technology was issued in 1912), but can not surprise that its wording played during the XX century and the other authors. Action on the world tendency to scattering of non-thermal forms of energy as heat all of them, since Ostwald correctly transformed into the law knows no exceptions.
Carnot cycle requires a fairly peculiar device: the cylinder and the piston are made of a perfect thermal insulator, but at the bottom of the cylinder can pass the heat. His alternately put on the heater, the fridge, then heat insulator. To ensure the equilibrium of the piston to move with an infinitely small velocity. Efficiency of any heat engine under the same heater and the refrigerator is less than the calculated value of Carnot. Illustration Oleg Sendyurev / "Around the World»

Refrigerator mandatory?
But back to the ban on thermal machines without refrigeration. Followers Carnot, abandoning caloric does not correct his mistake, in my opinion, because they worked exclusively with classical thermal machines with two features that make the fridge for them and indeed necessary: 1) cyclical; 2) a single-phase working fluid (gas or liquid). Returning a working fluid in the initial state, we have to give part of the heat received from the heater refrigerator. Meanwhile, the non-cyclic heat engines it is not necessary as it is not necessary, presumably, and cyclic heat engines with two-phase working fluid gas-liquid interface.
Examples of non-cyclic heat engine without a refrigerator can serve as working in a vacuum rocket engine for which talk about the cooling of the combustion products behind it is not necessary - the expansion of the gas in the void, as it is known, takes place isothermally. Another example of non-cyclic heat engine without a refrigerator is shown below.
As for the cyclic heat engines with two-phase working fluid, the way to prove it in the last decade independently several local researchers, the return of the working fluid in the initial state can they be carried out not with the transfer of the heat refrigerator, but on its return to the heater. Specifically, part of the useful work of one phase working fluid used for adiabatic expansion and hence cooling of the other. External refrigerator is unnecessary, and efficiency - not limited to the Carnot efficiency of the machine.
The second law of thermodynamics
A favorite argument of defenders of perpetual motion machine of the second kind - the limitations of the second law of thermodynamics. My position is different. I believe that the second - it is the law of nature acting, if we exclude the microscopic fluctuations, without restrictions. Another thing that should be properly read it.
In modern textbooks and academic monographs content of the second start too blurry, as evidenced by the diversity of its wording. I collect them for many years, and so far in my collection of 48 language, but in reality more of them. This diversity contrasts, for example, with the wording of the law of conservation of energy, which are in different sources almost word-for-word repeat each other.
We often read about the identity of the different formulations of the second law. This is both true and not. Of course, all of the many formulations of the second law can not be identical, but they can all be different. I tried vyshelushit core of the law of nature, which is behind all this, and I got two of these components: 1) the law of the increase of the total entropy; 2) there is an asymmetry between transformations nonthermal forms of energy into heat, on the one hand, and the conversion of heat into other forms of energy - the other: first, in contrast to the second, do not require compensation.
The most common source of confusion I see in the inability to distinguish between thermal and non-thermal entropy. Meanwhile, the difference between them is easy to demonstrate. If you mix the hot air with cold, we get warm air - the same thing that the transfer of a certain amount of heat from the hot air cold. Entropy has increased with the participation of the thermal process.
If the mix oxygen with hydrogen at the same temperature, the growth of entropy is not associated with the transfer of heat. When mixing cold with hot oxygen-hydrogen entropy is growing and thanks to equalize the temperature, and due to the simple mixing (diffusion). The problem is that under certain conditions the growth of the total entropy can be accompanied by reduction of one of the terms - for example, heat.
In general, the law increases the total entropy does not apply and the "law" of increasing the thermal entropy. Since thus the thermal entropy can decrease, so far as the conversion of heat into other forms of energy can occur with a decrease in thermal entropy, only to grow full. This means that the conversion of heat into other forms of energy can be full, i.e. to occur without the thermal compensation.
Crookes radiometer shows how thermal energy can be converted into mechanical in nonequilibrium systems. The bright and dark surfaces of the blades are heated by light incident upon them and, in turn, a different heated air in their vicinity. Where the temperature is higher and larger pressure - as a result of the blades begin to rotate. Photos (Creative Commons license): Leo-setä

Obligation of the refrigerator for any heat engine today to explain the need for increasing the thermal entropy. Cancel "law" does it increase the ban on perpetual motion machines of the second kind insolvent, paving the way for the creation of energy, built on the circulation of heat.
On the projects of perpetual motion machine of the second kind
Today, there are dozens of such projects. However, all of them indiscriminately and declared a priori contradictory second law of thermodynamics and therefore unworthy of critical analysis. As a result, the authors are forced to "cook" in your own environment, which, of course, prevent them from becoming the object of rational criticism and reduces the level of scientific texts, often - to unacceptably low. It is very difficult to separate the chaff from the rye. I'll tell only one such project, the idea of which seems to me quite worthy of discussion.
Put into the wind in the atmosphere of a tapered tube where the air is accelerated by "geometric" reasons, like the air in a crevice between rocks or in a narrow passage between the houses. Such a flow of an ideal gas approximation is described by Bernoulli's equation, known in two main forms. The first is that the acceleration of the gas along the line of current is accompanied by a decrease in its pressure, according to the second - a drop in temperature. The first effect of the wing provides the lift, the second, I think, can be laid in the foundation of a perpetual motion machine of the second kind.
In fact, the cooling gas flow means reducing the number of the contained heat acceleration - increase its kinetic energy. The thermal energy is directly converted into kinetic energy here, there is no refrigerator. The cooling gas flow takes place with decreasing thermal entropy, which is compensated by the growth of non-thermal entropy associated with a decrease in pressure.
Narrows the tube may be provided with a turbine, turning it into a power plant. In "Wind Turbine" this kind of Russian inventors received a patent Mikhail Yegorov, Igor Orlov and Emmanuel Avraamovich Sable. Their installation looks in the drawings as a pot-bellied bomb hanging along the airflow and accept it inward annular hole.
The reader has the necessary experimental base (What is missing from the author), he can put the experimentum crucis, using, for example, for the construction of a tapered tube film for greenhouses, secured with steel wire frame.
Installing Egorova-Orlova-Sable, it seems to me, can be adapted to the aquatic environment where it can have a greater capacity per unit volume as the waters of the earth contains much more heat than the atmosphere per unit volume.
But it is not in fact whether this particular design. My tasks included the presentation of projects is not perpetual motion machine of the second kind, which could be run immediately into production. I'm just trying to reverse a steady negative attitude of Big Science to the very idea of such engines.
Sergei Haytun,
www.vokrugsveta.ru/telegraph/theory/1185
Fan Dyson works without blades. It looks strange, but there is no contradiction to the laws of physics! Photo: Dyson

Source: