494
The electron microscope has discovered how vitamin a enters the cells

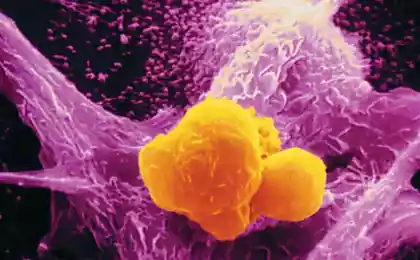
Using the new, high speed camera paired up with an electron microscope, scientists at Columbia University have captured images of one of the smallest proteins in our cells to "see" it with a microscope.
A protein called STRA6 is located in the membrane of our cells and responsible for transporting vitamin A into the cells. Vitamin a is essential for all mammals and is particularly important in the formation of the light receptors in our eyes during the embryonic period, it is crucial for normal development.
To new research, as STRA6 transports vitamin a in the cage was a mystery. Most transport proteins directly interact with substances they carry. But STRA6 interacts with vitamin a through intermediary protein that carries vitamin a in the blood. Continuation under the cut...
A new type of camera technology is a key element to obtain images of STRA6. In combination with an electron microscope, the camera allows biologists to see a tiny, previously non-visible structural details of the internal mechanism of our cells.
"Now we can approach atomic resolution, because the new camera is much faster and allows us to create a film of molecules," says Oliver Clark, Ph. D., adjunct research scholar in the lab of Hendrickson at Columbia University. "Even under an electron microscope, the molecules move a little bit, but when we took a picture of something moving, it came out blurry. Even with such images we can align frames to create a clearer image.
Visualization of the molecules also depends on laborious biochemical procedures developed by Yanting Chen, PhD, adjunct research fellow in the laboratory of Mancia to produce large amounts of protein and separate it from other components of the cell. "This is a very delicate protein, and we had to mimic its environment to preserve its form," she says. Took about two years to perfect the process of obtaining.
The researchers used about 70,000 individual photos STRA6 to create a 3D map of the protein that was used to build the model of the atom with precision to the smallest detail.
Image the molecules still look STRA6 is "a bit ugly", says Dr Clark. Even more surprising is the fact that STRA6 is not working alone, but instead it is tightly associated with another protein, calmodulin, which plays a key role in the transmission of calcium signals.
Although vitamin a is moving via STRA6 to enter the cage STRA6 does not form channels in the membrane as the most of transport proteins. Instead, the vitamin A enters the upper part of STRA6, but then produces a window that opens directly to the cell membrane and not inside the cell.
Although it is proven that this mechanism could be a way to protect cells from too much vitamin A. He "is actually toxic," says Dr. Manche. "Traps of vitamin A inside the membrane can be kept under the control of his number inside the cell".
A new model of STRA6 increases understanding cellular functions and could help scientists understand how other, still unknown cellular components.
Source: newsroom.cumc.columbia.edu
Source: geektimes.ru/company/scione/blog/280310/