468
The hydrogen from water, rust and sunlight
Preservation and convenient storage of power generated from solar batteries, the problem is no less urgent than the efficiency of photoelectric transformation. How to ensure the availability of energy at any time of the day or night?
Researchers from the Federal Polytechnic school in Lausanne (École polytechnique fédérale de Lausann, EPFL) are developing a technology that can transform solar energy into hydrogen, a clean fuel with a neutral carbon footprint.
Ingredients process the most simple and common, water and iron oxide or ordinary rust. Kevin Sivula (Kevin Sivula), together with colleagues intentionally limited to cheap and common, easily recoverable materials to obtain a viable and inexpensive method of producing solar hydrogen. Their device is still in the experimental stage, but about it already wrote in the journal Nature Photonics.
"The most expensive material in our plant – glass plate," explains Sivula. The efficiency of the device remains low, from 1.4 to 3.6%. But technology great potential. "With our low-cost concept on the basis of iron oxide, we hope to achieve efficiency 10% within a few years at a cost of less than $80 per square meter [of the working surface of the device]. At this price we will be able to compete with traditional methods of hydrogen production".
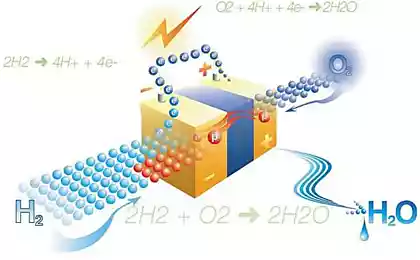
The idea is not new. Working on it various scientists for more than 40 years. Fully self-contained device, consisting of two layers, the electrons emitted oxide semiconductor under the action of sunlight is used to split water molecules into oxygen and hydrogen. Hydrogen is extracted by means of cells with dye-sensitized.
The semiconductor in this case is the usual rust. "It is a stable and common material, it will not rust any further! But this is one of the worst semiconductors available," admits Sivula. To improve the properties of material, scientists enrich it with nanostructured silicon oxide and coated with a thin layer of aluminum oxide and cobalt.
The second layer of the working surface of the device consists of a dye and titanium dioxide. It allows us to give a dedicated semiconductor the electrons enough energy to extract hydrogen from water.
According to the researchers, their findings they were able to achieve through the use of the latest advances in the study of iron oxide and titanium dioxide. The theoretical limit of efficiency of their technology can be up to 16% without significantly increasing cost. Perhaps, over time, such systems can greatly increase the potential of solar energy.
Source: /users/104
Researchers from the Federal Polytechnic school in Lausanne (École polytechnique fédérale de Lausann, EPFL) are developing a technology that can transform solar energy into hydrogen, a clean fuel with a neutral carbon footprint.
Ingredients process the most simple and common, water and iron oxide or ordinary rust. Kevin Sivula (Kevin Sivula), together with colleagues intentionally limited to cheap and common, easily recoverable materials to obtain a viable and inexpensive method of producing solar hydrogen. Their device is still in the experimental stage, but about it already wrote in the journal Nature Photonics.
"The most expensive material in our plant – glass plate," explains Sivula. The efficiency of the device remains low, from 1.4 to 3.6%. But technology great potential. "With our low-cost concept on the basis of iron oxide, we hope to achieve efficiency 10% within a few years at a cost of less than $80 per square meter [of the working surface of the device]. At this price we will be able to compete with traditional methods of hydrogen production".

The idea is not new. Working on it various scientists for more than 40 years. Fully self-contained device, consisting of two layers, the electrons emitted oxide semiconductor under the action of sunlight is used to split water molecules into oxygen and hydrogen. Hydrogen is extracted by means of cells with dye-sensitized.
The semiconductor in this case is the usual rust. "It is a stable and common material, it will not rust any further! But this is one of the worst semiconductors available," admits Sivula. To improve the properties of material, scientists enrich it with nanostructured silicon oxide and coated with a thin layer of aluminum oxide and cobalt.
The second layer of the working surface of the device consists of a dye and titanium dioxide. It allows us to give a dedicated semiconductor the electrons enough energy to extract hydrogen from water.
According to the researchers, their findings they were able to achieve through the use of the latest advances in the study of iron oxide and titanium dioxide. The theoretical limit of efficiency of their technology can be up to 16% without significantly increasing cost. Perhaps, over time, such systems can greatly increase the potential of solar energy.
Source: /users/104