385
The sodium battery will help to desalinate the water
A lot of scientists struggling to resolve two intractable problems: the provision of energy and clean water future world. But what if both tasks can be solved with a single technology?
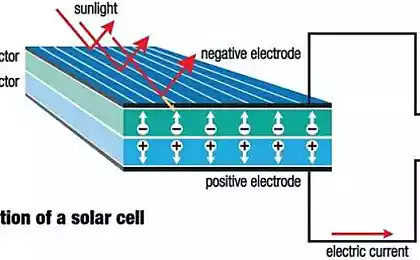
Technology that charges batteries for electronic devices can also provide fresh water obtained from sea salt, says a new study by engineers at the University of Illinois. Electricity passes through the water-filled sodium battery and removes salt ions from the water.
Professor of mechanics and engineering of the University of Illinois ' Kyle Smith (Kyle Smith) and graduate student, Dmello Rylan (Rylan Dmello) published his work in journal of the Electrochemical Society.

"We are developing a device that will use the materials in the batteries for the withdrawal of salt from the water with the least amount of energy possible," said Smith.
The interest in desalination technologies is growing as water demand, especially in arid areas. However, countries are faced with technical obstacles and the need to use huge amounts of energy, that is what prevents large scale using various technologies.
The most used method, reverse osmosis pushes water through a membrane that retains the salt, but it's expensive and energy-intensive process. In contrast, the method uses the battery power to output the charged salt ions from water.
The researchers were inspired by the sodium-ion batteries, which contain salt water. Batteries have two compartment, the positive electrode and negative electrode with separator between them, through which ions can move. If the battery discharges, the sodium and chloride ions are two elements of salt — are drawn to one compartment, leaving desalinated water in the other.
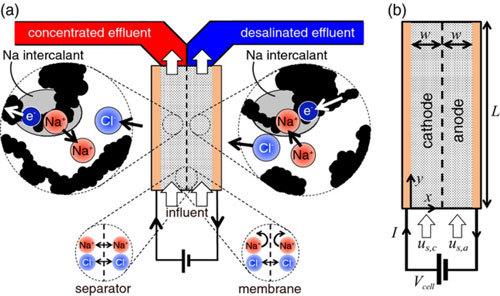
In a normal battery, the ions diffuse back when the current flows in the opposite direction. Researchers at the University of Illinois have found a way to keep the salt separate from the water.
"In a conventional battery separator allows the salt to diffuse from the positive electrode to the negative," explains Smith. "This limits the amount of salt that can be allocated. We put a membrane that blocks the sodium between the two electrodes, so we can keep it from the part where there is water."
Smith and Mello conducted a study on the model to see how their device can work with water, where a higher salt concentration than sea water, and found that it can provide about 80 percent of desalinated water. Their modeling does not account for other contaminants in water, so they work on experiments with real seawater.
"We believe that the technology is promising," said Smith. "Of course, there are a lot of work, we need to develop new materials for sodium-ion battery. I hope our work will encourage scientists in this region for the study of new materials for desalination".
published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: facepla.net/the-news/tech-news-mnu/5373-%D0%BE%D0%BF%D1%80%D0%B5%D1%81%D0%BD%D1%8F%D1%82%D1%8C-%D0%B2%D0%BE%D0%B4%D1%83.html
Technology that charges batteries for electronic devices can also provide fresh water obtained from sea salt, says a new study by engineers at the University of Illinois. Electricity passes through the water-filled sodium battery and removes salt ions from the water.
Professor of mechanics and engineering of the University of Illinois ' Kyle Smith (Kyle Smith) and graduate student, Dmello Rylan (Rylan Dmello) published his work in journal of the Electrochemical Society.

"We are developing a device that will use the materials in the batteries for the withdrawal of salt from the water with the least amount of energy possible," said Smith.
The interest in desalination technologies is growing as water demand, especially in arid areas. However, countries are faced with technical obstacles and the need to use huge amounts of energy, that is what prevents large scale using various technologies.
The most used method, reverse osmosis pushes water through a membrane that retains the salt, but it's expensive and energy-intensive process. In contrast, the method uses the battery power to output the charged salt ions from water.
The researchers were inspired by the sodium-ion batteries, which contain salt water. Batteries have two compartment, the positive electrode and negative electrode with separator between them, through which ions can move. If the battery discharges, the sodium and chloride ions are two elements of salt — are drawn to one compartment, leaving desalinated water in the other.

In a normal battery, the ions diffuse back when the current flows in the opposite direction. Researchers at the University of Illinois have found a way to keep the salt separate from the water.
"In a conventional battery separator allows the salt to diffuse from the positive electrode to the negative," explains Smith. "This limits the amount of salt that can be allocated. We put a membrane that blocks the sodium between the two electrodes, so we can keep it from the part where there is water."
Smith and Mello conducted a study on the model to see how their device can work with water, where a higher salt concentration than sea water, and found that it can provide about 80 percent of desalinated water. Their modeling does not account for other contaminants in water, so they work on experiments with real seawater.
"We believe that the technology is promising," said Smith. "Of course, there are a lot of work, we need to develop new materials for sodium-ion battery. I hope our work will encourage scientists in this region for the study of new materials for desalination".
published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: facepla.net/the-news/tech-news-mnu/5373-%D0%BE%D0%BF%D1%80%D0%B5%D1%81%D0%BD%D1%8F%D1%82%D1%8C-%D0%B2%D0%BE%D0%B4%D1%83.html
Self-contained mini-house in the mountains of the legendary snowboarder Mike Basic
The broken Windows theory: how to win the crime in new York