461
Developed stable chemical formula of a flow battery
Scientists at the universities of Utah and Michigan have developed a chemical formula for flow redox battery, 1000 times more stable than modern counterparts.
All batteries are chemical substances that store and release electrical charge. However, flow redox batteries (working on the oxidation of-recovery) are not like batteries in cars or smartphones. They have two containers for storing the energy separated by a set of inert electrodes.

Modern flow redox batteries operate on a solution of vanadium, a valuable substance that needs special care because of its potential toxicity. Creating formulas like this requires a battery of chemical equilibrium, because the molecules capable of storing more energy, for example, generated by solar panels, are less stable.
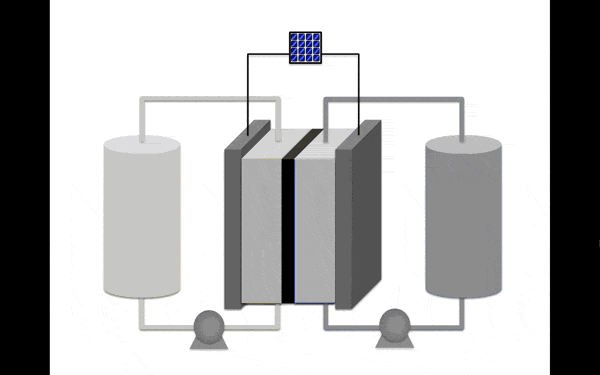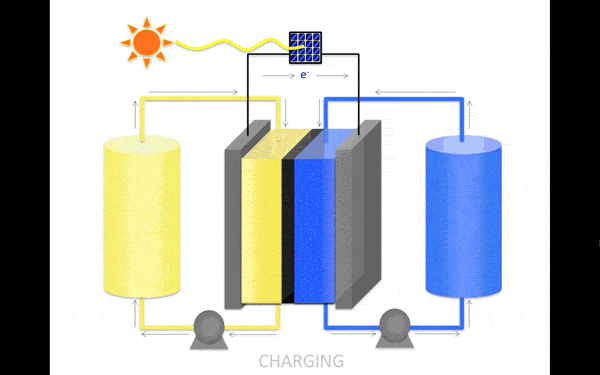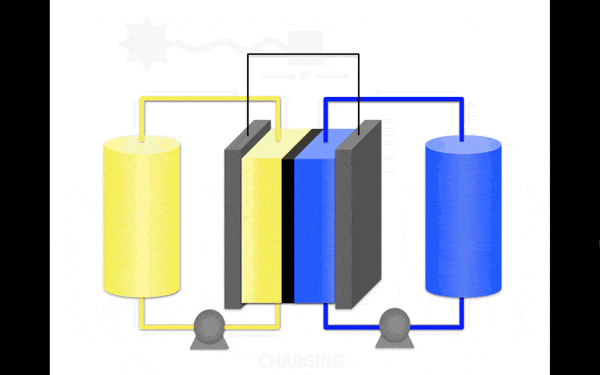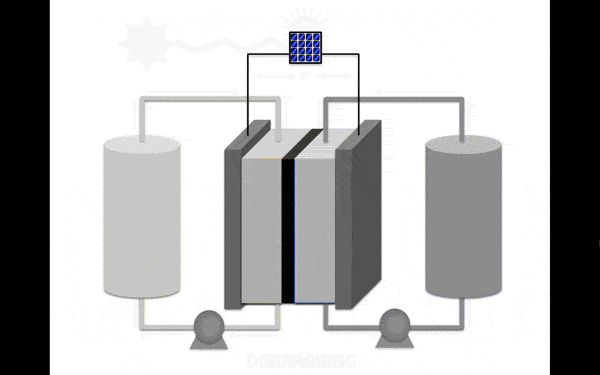
The most promising material for the anolyte (the portion of the electrolyte closest to the anode) became an organic molecule pyridine. It contains no metals and is soluble in organic solution, increasing its stability. Scientists discovered that the molecules in the proposed electrolyte disintegrate when interacting with each other. But if you stop them to meet, then the collapse occurs.
Usually the half-life of the molecules of the electrolyte of the battery is 8-12 hours, said Matthew Sigman, one of the authors of an article published in the Journal of the American Chemical Society, "and the structure that we predicted is stable for months."
Now scientists are trying to find the catholyte (part of the electrolyte closest to the cathode) to combine it with this molecule and lay the Foundation of the structure of the new flow-through battery. "It's a multistep process, but it will not work unless you have a stable molecules with low redox potential. The job needs to be here," said Melanie Sanford from the University of Michigan.
A new kind of flow battery were presented by the chemists of the University of Pennsylvania. It is possible to charge an aqueous solution of carbon dioxide. The device generates electricity due to the difference of CO2 concentrations in emissions and the air. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hightech.fm/2017/02/24/redox-molecule
All batteries are chemical substances that store and release electrical charge. However, flow redox batteries (working on the oxidation of-recovery) are not like batteries in cars or smartphones. They have two containers for storing the energy separated by a set of inert electrodes.

Modern flow redox batteries operate on a solution of vanadium, a valuable substance that needs special care because of its potential toxicity. Creating formulas like this requires a battery of chemical equilibrium, because the molecules capable of storing more energy, for example, generated by solar panels, are less stable.

The most promising material for the anolyte (the portion of the electrolyte closest to the anode) became an organic molecule pyridine. It contains no metals and is soluble in organic solution, increasing its stability. Scientists discovered that the molecules in the proposed electrolyte disintegrate when interacting with each other. But if you stop them to meet, then the collapse occurs.
Usually the half-life of the molecules of the electrolyte of the battery is 8-12 hours, said Matthew Sigman, one of the authors of an article published in the Journal of the American Chemical Society, "and the structure that we predicted is stable for months."
Now scientists are trying to find the catholyte (part of the electrolyte closest to the cathode) to combine it with this molecule and lay the Foundation of the structure of the new flow-through battery. "It's a multistep process, but it will not work unless you have a stable molecules with low redox potential. The job needs to be here," said Melanie Sanford from the University of Michigan.
A new kind of flow battery were presented by the chemists of the University of Pennsylvania. It is possible to charge an aqueous solution of carbon dioxide. The device generates electricity due to the difference of CO2 concentrations in emissions and the air. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: hightech.fm/2017/02/24/redox-molecule
The law in California will make Lancaster the first in the United States with zero energy consumption
Innovative power plant operates without CO2 emissions