511
Organic batteries of the future
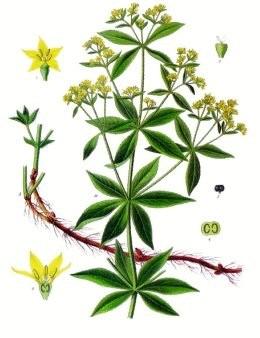
The madder plant or Rubia tinctorum is a great source of organic dye purpurin, long used by the people for dyeing. Scientists from rice University and City College of new York found that the advantages of the pigment is not limited to its color. The purpurin can be used as effective, natural cathode for lines-ion batteries.

Getting to the research, scientists from rice hoped to find ways to improve the properties of the common lithium-ion batteries. In their opinion, the substance of plant origin may serve as a basis for environmentally sound storage of electricity and to solve many significant problems.
As noted by lead author arava Lime Mohan Reddy (Arava Leela Mohana Reddy): "Green batteries need today, but until now this topic was not addressed properly. Currently, the activities of the scientific community is still focused on conventional batteries, mostly on the increase of their capacity. At the same time, issues such as recycling and sustainability are critical."
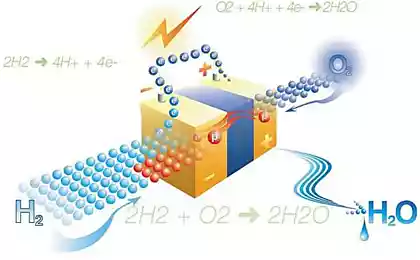
Lithium-ion batteries have become standard. However, as before, they remain expensive to produce. In addition, according to Reddy, they are a potential threat to the environment. "They use cathodes of lithium and cobalt oxide, which are very expensive. Mining and production of cobalt cathodes in high temperature environment is costly. The big problem is processing. In 2010, the processing required almost 10 billion lithium-ion batteries, which requires a lot of energy. Extract from batteries, cobalt is an expensive process."
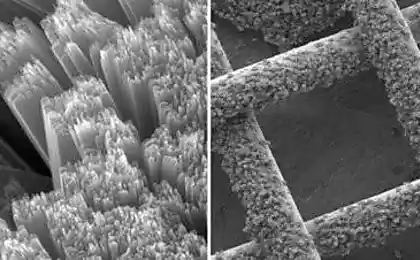
Reddy and his colleagues came across purpurin while testing organic substances on the ability of electrochemical interaction with lithium. It turned out that the pigment is most suitable for binding lithium ions.
Adding to the purpurin 20% carbon, which increased electrical conductivity, scientists have built half of the battery specific capacity of 90 mA/h per gram after 50 charge-discharge cycles. The fabrication of the cathode occurred at room temperature.
According to Reddy, they managed to develop a very simple mechanism. Presumably the raw material for the cathodes of new type can be agricultural wastes, which will make the technology even more economical. Thus, innovative batteries solve two problems, the need for efficient storage of electric power and recycling.
As noted by another lead author, Professor of chemistry City College of new York George John (John George): "the Problem was to understand the chemistry of the interaction between lithium ions and organic molecules. Now we have correct understanding, and we can use other molecules to improve the process".
Scientists seek to create a fully green battery. The team is looking for organic molecules suitable for anodes and electrolyte. Hopes Reddy, a working prototype of a complete organic battery can occur within a few years.
Source: /users/276

Getting to the research, scientists from rice hoped to find ways to improve the properties of the common lithium-ion batteries. In their opinion, the substance of plant origin may serve as a basis for environmentally sound storage of electricity and to solve many significant problems.
As noted by lead author arava Lime Mohan Reddy (Arava Leela Mohana Reddy): "Green batteries need today, but until now this topic was not addressed properly. Currently, the activities of the scientific community is still focused on conventional batteries, mostly on the increase of their capacity. At the same time, issues such as recycling and sustainability are critical."
Lithium-ion batteries have become standard. However, as before, they remain expensive to produce. In addition, according to Reddy, they are a potential threat to the environment. "They use cathodes of lithium and cobalt oxide, which are very expensive. Mining and production of cobalt cathodes in high temperature environment is costly. The big problem is processing. In 2010, the processing required almost 10 billion lithium-ion batteries, which requires a lot of energy. Extract from batteries, cobalt is an expensive process."

Reddy and his colleagues came across purpurin while testing organic substances on the ability of electrochemical interaction with lithium. It turned out that the pigment is most suitable for binding lithium ions.
Adding to the purpurin 20% carbon, which increased electrical conductivity, scientists have built half of the battery specific capacity of 90 mA/h per gram after 50 charge-discharge cycles. The fabrication of the cathode occurred at room temperature.
According to Reddy, they managed to develop a very simple mechanism. Presumably the raw material for the cathodes of new type can be agricultural wastes, which will make the technology even more economical. Thus, innovative batteries solve two problems, the need for efficient storage of electric power and recycling.
As noted by another lead author, Professor of chemistry City College of new York George John (John George): "the Problem was to understand the chemistry of the interaction between lithium ions and organic molecules. Now we have correct understanding, and we can use other molecules to improve the process".
Scientists seek to create a fully green battery. The team is looking for organic molecules suitable for anodes and electrolyte. Hopes Reddy, a working prototype of a complete organic battery can occur within a few years.
Source: /users/276