1228
At Stanford figured out how to increase the capacity of lithium-ion batteries in 3-4 times using carbon nanospheres

In contemporary lithium-ion batteries the anode is typically a graphite layer is deposited on the copper foil. During the charge in a layer of graphite, lithium ions are accumulated. This design was invented for reasons of safety and durability - the anode of pure lithium metal is very rapidly degraded, since the charge on the surface gradually grow "branches" that by reaching the cathode, can cause a short circuit. Using graphite "sponge" solves this problem, but power consumption of the graphite anode is about 350 milliamp hours per gram, whereas the rate of pure lithium up to 11 times.
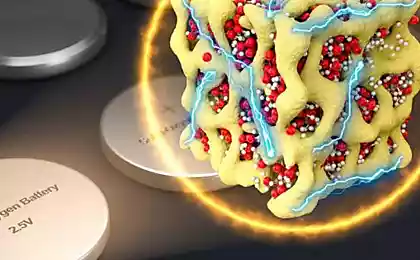
A group of scientists at Stanford University, led by professor and Chui (Yi Cui) разработали technology , which prevents the formation of "branches" at the anode of a lithium metal and opens the way for the establishment of reliable and much more capacious battery. In practice, we can talk about increasing capacity in three or four times.
A key element of the new technology - creating on the surface of the anode of the finest 20-nanometer layers of interconnected carbon nanospheres. This layer is created by applying an electrode layer of tiny polystyrene beads which spontaneously form a regular structure resembling a honeycomb. This layer serves as the foundation on which is then applied to carbon. Then polystyrene is removed by heating in an argon atmosphere, and remains on the surface of the electrode thin but durable coating of carbon honeycomb. Through this coating freely penetrate the lithium ions, but it is strong enough to stop the growth of lithium "branches».

Above - the charge-discharge cycles the battery coated anode and without it, the bottom - a coating scheme. I>
Выход current battery electrode reaches a new type of 99% even after 150 cycles of charging, while the lithium electrode with no protective coating it falls from 96% at the start of operation up to less than 50% after 100 cycles, which makes a battery completely impractical. According And Chewie, further improvements, in particular the selection of suitable types of electrolytes, may soon bring the current output to 99, 9% - at this level is already possible to speak about the commercial use of the technology.
Source: habrahabr.ru/post/231397/
5 signs of a good illustration for kids
In NASA tested the prototype laser drilling of ice in Europe, Jupiter's moon