482
How to understand the behavior of an electron
Thirty five million four hundred thirty one thousand eight hundred twenty nine
I want to tell you about one of the most beautiful ideas. This physical experiment, and it's beautiful because in one elegant motion, he expands our consciousness, forcing us to realize that objects can behave in ways that we cannot imagine. But remarkably, it can be calculated. It's beautiful, because it casts doubt on the fundamental principles of logic on which we have built our understanding of the world. Perfect because it is deceptively simple to understand, but its consequences are frightening. Hopefully, your picture of the world will be destroyed. Further — the text of the first-person narrator with Wired.
It was eleven years ago. I was a College freshman, sitting in the physical laboratory, turning off the light, and staring at a blank computer screen. In the background played the hits of the 80's.
So, I've been given. On the table in front of me was a box with two thin slit-like openings on one end. We're shooting particles into this box through the gap. I conducted an experiment with photons, particles of light, but you can hold it with electrons and, in principle, with anything. It could even be a ball of 60 carbon atoms, which is huge compared to electrons. For convenience, I'll call the objects of the experiment with electrons, but keep in mind that it can be any material that is broken into pieces.
At the other end of the box is a CCD camera which takes a picture when something strikes her. Each time a particle passes through the box, I see the relevant point lights up on the screen of my computer.
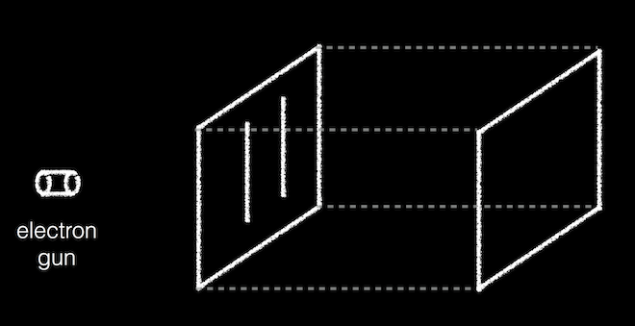
For greater caution we set up the experiment so that at any given time inside the box there can be only one particle. Imagine a very tiny ball thrown into the box. Music plays, we sit and wait.
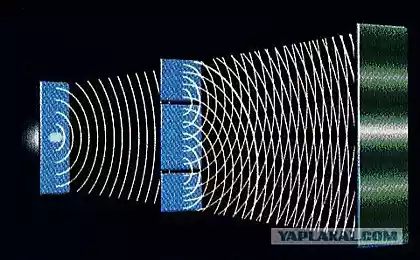
What would you expect to see on the other end of the box? If electrons behave as waves, you will see bright and dark bands, like ripples in a water tank. This happens because the waves interfere with each other, are extinguished when the peak of one meets another wave, and reinforced when the peaks line up.
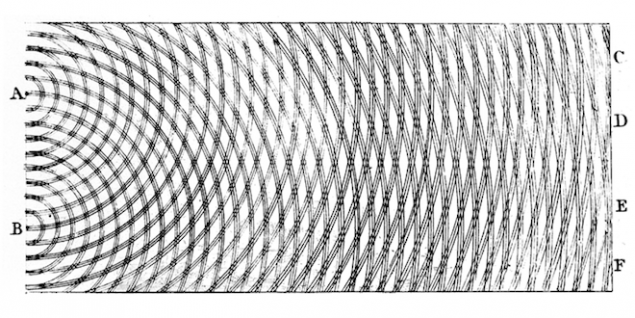
But the electrons are not waves, it's whole pieces. I know this because I see it as they arrive on the screen one by one and beat in one place, like rain on asphalt. And if the electrons are the solid pieces, you will only see them piling up behind the gap and nowhere else. In short, you expect that they will behave like balls.
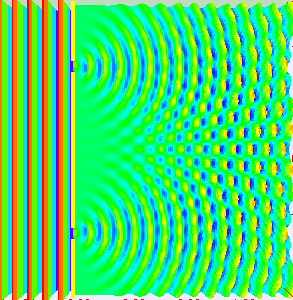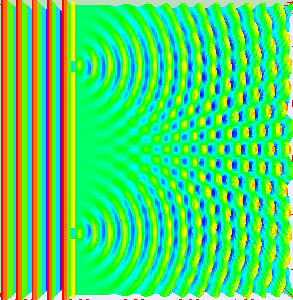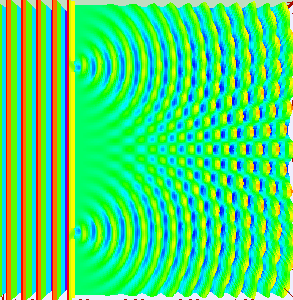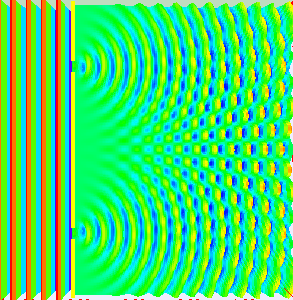
Really, if you are conducting the experiment with only one slit open, they behave like the balls, getting into a strict band behind the open slit. It is reasonable to assume that when we open both slits, we will see two lanes, one corresponding to each slit.
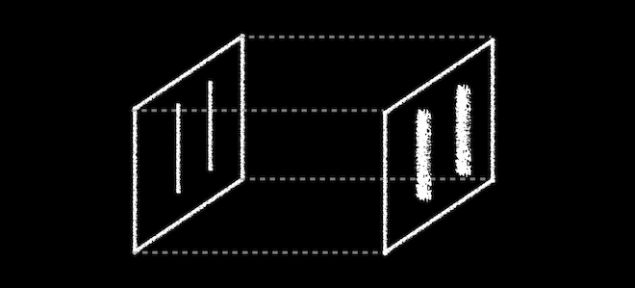
What do the electrons?See for yourself. In this video, made by scientists at Hitachi in 1989 year, you can see that they do electrons. Video sped up 30 times.
Need time to understand how it's weird. Somehow, the electrons created this interference pattern of bright and dark bands. But they went one by one, as they could interfere with each other? If you imagine an electron in a tiny ball, you have to conclude that the electron passing through one slit, and slips through the other. He chooses both paths and interferes with itself. Complete nonsense.
Let's go back and try to collect all the data. Born obvious question to ask. Think of the electrons that hit the screen. Through which slit did it go?
Through the left?No. Because when you cover the right slit, the striped pattern disappears and you're left with a boring one strip.
Through the right?No. For the same reason as above. If you cover up the left slit, you'll get a boring bar.
Through both?No. Because if that were true, we would expect to see the electron is divided into two parts, and one electron (or half of it) goes through each slit. But if you place detectors at the slits you find that this is not happening. You will always see only one electron at a time. He never breaks down into two parts.
Generally goes nowhere?No. Of course not, what nonsense. If you cover both slits, nothing happens.
At this point you begin to think that all this is getting a little ridiculous. Why can't we just follow the damn electron and see which slit it goes through? The problem is that looking at something means to highlight it, as if to illuminate the electron, this means to push it with the photon. If you are a tiny electron, such a blow would knock you out of the way.
But wait. Maybe if you make a shot is very very soft, you will not be disturbed electron? The thing is that if you make the light more gentle (lower momentum), you will make it more diffused (increase the wavelength) and in the end will not be able to tell through which slit the electron passed.
It's a dead end. Any scheme you can come up with to define the route of the electron destroys the interference pattern.
Summing up, we came to the striped pattern which is generated by any particle. But if you're trying to find out exactly how the particle is on the wall, you come to the conclusion that she doesn't choose the left route, don't choose the right route, do not choose both ways and refuses both. As noted by mit Professor Allan Adams is largely exhausts all logical possibilities.
Electron is not like a wave because, unlike the wave hits the screen at one point. Electron is not like a ball because if you throw it through a double slit, it interferes and forms a stripe pattern. There is no analogy that will help you to understand what electron. It's fucking magic.
As he said in his lectures on quantegy Allan Adams:
! These electrons are doing something we never thought of before, what never dreamed of, as even the appropriate words in the language there.!
It turns out that electrons have an empirical way of movement and existence that is different from what we are accustomed to. Like molecules. And bacteria. These objects are just difficult to detect. Physics came up with the name of this model of existence. We call it superposition.
Sometimes it is useful to think about electron as a particle, it is sometimes useful to think of it as a wave. But it's only convenient for our speech, and both ways of naming incomplete. The electron is not a wave and not a particle. Electron is an electron. The same applies to the photon, atom, ball or giant molecule, there you have it. The larger the object the harder it is to see these bands.
Werner Heisenberg, one of the founders of quantum mechanics, is well understood. In 1930, he wrote:
"The problem is that the two mental pictures which helps us experiment — one particle and one a wave is both incomplete and have only approximate analogies which are accurate only in certain cases. The apparent duality arises only because of the limitations of our language."
As taught by Heisenberg and others, although language fails us, we can come up with rules that accurately explain how tiny things behave. These rules are quantum mechanics. Using these rules, physicists can throw complex phrases like "the electron wave function is in superposition and passes through the left and right slit". These proposals very accurately explains the mathematical expressions, and based on them made the most accurate experiments. Only lacks a coherent picture, which you can fold in my head and that will explain which path the electron chooses. Moreover, we are pretty sure that this picture will never develop.
There is nothing surprising in the fact that our monkey brains that evolved, throwing spears and stones of medium size, are unable to visualize the behavior of very small things. But what's even more surprising is that even though we can't visualize the quantum world, we managed to develop rules of the game.
Source: hi-news.ru
I want to tell you about one of the most beautiful ideas. This physical experiment, and it's beautiful because in one elegant motion, he expands our consciousness, forcing us to realize that objects can behave in ways that we cannot imagine. But remarkably, it can be calculated. It's beautiful, because it casts doubt on the fundamental principles of logic on which we have built our understanding of the world. Perfect because it is deceptively simple to understand, but its consequences are frightening. Hopefully, your picture of the world will be destroyed. Further — the text of the first-person narrator with Wired.
It was eleven years ago. I was a College freshman, sitting in the physical laboratory, turning off the light, and staring at a blank computer screen. In the background played the hits of the 80's.
So, I've been given. On the table in front of me was a box with two thin slit-like openings on one end. We're shooting particles into this box through the gap. I conducted an experiment with photons, particles of light, but you can hold it with electrons and, in principle, with anything. It could even be a ball of 60 carbon atoms, which is huge compared to electrons. For convenience, I'll call the objects of the experiment with electrons, but keep in mind that it can be any material that is broken into pieces.
At the other end of the box is a CCD camera which takes a picture when something strikes her. Each time a particle passes through the box, I see the relevant point lights up on the screen of my computer.

For greater caution we set up the experiment so that at any given time inside the box there can be only one particle. Imagine a very tiny ball thrown into the box. Music plays, we sit and wait.
What would you expect to see on the other end of the box? If electrons behave as waves, you will see bright and dark bands, like ripples in a water tank. This happens because the waves interfere with each other, are extinguished when the peak of one meets another wave, and reinforced when the peaks line up.

But the electrons are not waves, it's whole pieces. I know this because I see it as they arrive on the screen one by one and beat in one place, like rain on asphalt. And if the electrons are the solid pieces, you will only see them piling up behind the gap and nowhere else. In short, you expect that they will behave like balls.

Really, if you are conducting the experiment with only one slit open, they behave like the balls, getting into a strict band behind the open slit. It is reasonable to assume that when we open both slits, we will see two lanes, one corresponding to each slit.

What do the electrons?See for yourself. In this video, made by scientists at Hitachi in 1989 year, you can see that they do electrons. Video sped up 30 times.
Need time to understand how it's weird. Somehow, the electrons created this interference pattern of bright and dark bands. But they went one by one, as they could interfere with each other? If you imagine an electron in a tiny ball, you have to conclude that the electron passing through one slit, and slips through the other. He chooses both paths and interferes with itself. Complete nonsense.
Let's go back and try to collect all the data. Born obvious question to ask. Think of the electrons that hit the screen. Through which slit did it go?
Through the left?No. Because when you cover the right slit, the striped pattern disappears and you're left with a boring one strip.
Through the right?No. For the same reason as above. If you cover up the left slit, you'll get a boring bar.
Through both?No. Because if that were true, we would expect to see the electron is divided into two parts, and one electron (or half of it) goes through each slit. But if you place detectors at the slits you find that this is not happening. You will always see only one electron at a time. He never breaks down into two parts.
Generally goes nowhere?No. Of course not, what nonsense. If you cover both slits, nothing happens.
At this point you begin to think that all this is getting a little ridiculous. Why can't we just follow the damn electron and see which slit it goes through? The problem is that looking at something means to highlight it, as if to illuminate the electron, this means to push it with the photon. If you are a tiny electron, such a blow would knock you out of the way.
But wait. Maybe if you make a shot is very very soft, you will not be disturbed electron? The thing is that if you make the light more gentle (lower momentum), you will make it more diffused (increase the wavelength) and in the end will not be able to tell through which slit the electron passed.
It's a dead end. Any scheme you can come up with to define the route of the electron destroys the interference pattern.
Summing up, we came to the striped pattern which is generated by any particle. But if you're trying to find out exactly how the particle is on the wall, you come to the conclusion that she doesn't choose the left route, don't choose the right route, do not choose both ways and refuses both. As noted by mit Professor Allan Adams is largely exhausts all logical possibilities.
Electron is not like a wave because, unlike the wave hits the screen at one point. Electron is not like a ball because if you throw it through a double slit, it interferes and forms a stripe pattern. There is no analogy that will help you to understand what electron. It's fucking magic.
As he said in his lectures on quantegy Allan Adams:
! These electrons are doing something we never thought of before, what never dreamed of, as even the appropriate words in the language there.!
It turns out that electrons have an empirical way of movement and existence that is different from what we are accustomed to. Like molecules. And bacteria. These objects are just difficult to detect. Physics came up with the name of this model of existence. We call it superposition.
Sometimes it is useful to think about electron as a particle, it is sometimes useful to think of it as a wave. But it's only convenient for our speech, and both ways of naming incomplete. The electron is not a wave and not a particle. Electron is an electron. The same applies to the photon, atom, ball or giant molecule, there you have it. The larger the object the harder it is to see these bands.
Werner Heisenberg, one of the founders of quantum mechanics, is well understood. In 1930, he wrote:
"The problem is that the two mental pictures which helps us experiment — one particle and one a wave is both incomplete and have only approximate analogies which are accurate only in certain cases. The apparent duality arises only because of the limitations of our language."
As taught by Heisenberg and others, although language fails us, we can come up with rules that accurately explain how tiny things behave. These rules are quantum mechanics. Using these rules, physicists can throw complex phrases like "the electron wave function is in superposition and passes through the left and right slit". These proposals very accurately explains the mathematical expressions, and based on them made the most accurate experiments. Only lacks a coherent picture, which you can fold in my head and that will explain which path the electron chooses. Moreover, we are pretty sure that this picture will never develop.
There is nothing surprising in the fact that our monkey brains that evolved, throwing spears and stones of medium size, are unable to visualize the behavior of very small things. But what's even more surprising is that even though we can't visualize the quantum world, we managed to develop rules of the game.
Source: hi-news.ru
Scientists have created the illusion of invisibility
The tomb in Amphipolis was younger than Alexander the great