1552
Music theory and quantum mechanics

Note. pens. 1 (of music visualization techniques), 2 (about the basics of the conversion of analog to digital sound). i>
In high school, you probably saw a similar picture:
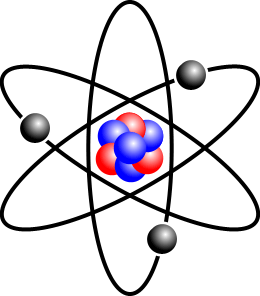
This figure shows a stylized red nucleus with protons and neutrons, blue, gray surrounded by three electrons. This pretty standard picture. From it can get a good logo. Unfortunately, she, at the same time, it is absolutely wrong. Subatomic particles to a certain extent like small glass beads, but the degree of similarity is very low. Electrons move around the nucleus really, but this is not the movement along an elliptical path, as if they were small satellites orbiting the planet. The true nature of the electrons in the atom is much more unusual and interesting. And the image can hardly convey the essence of quantum particles. Using the theory of music is made much easier.