285
How solar panels work

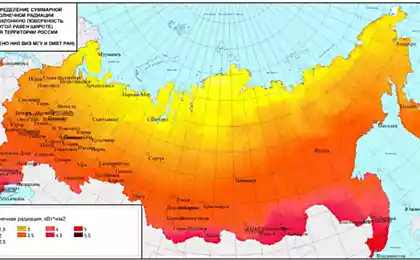
©Flickr/O'Connor College of Law Pix Solar power already covers more than 50% of Germany's energy costs. Obviously, the future of energy is solar panels. What are the basic principles of their work? At one time, solar cells were used almost exclusively in space, for example, as the main source of power for satellites. Since then, solar panels have increasingly entered our lives: they cover the roofs of houses and cars, are used in wristwatches and even in dark glasses. But how do solar panels work? How is it possible to convert the energy of sunlight into electricity?
Basic principles
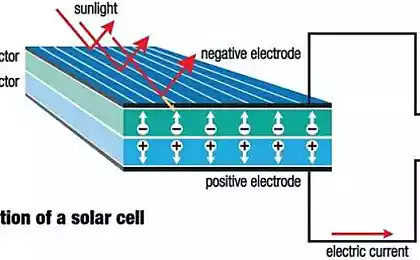
Solar panels consist of photovoltaic cells packed in a common frame. Each is made of a semiconductor material, such as silicon, which is most commonly used in solar panels.
When the rays fall on the semiconductor, it heats up, partially absorbing their energy. The inflow of energy releases electrons inside the semiconductor. An electric field is attached to the solar cell, which directs free electrons, causing them to move in a certain direction. This flow of electrons forms an electric current.
If you apply metal contacts to the top and bottom of the photocell, you can direct the resulting current through the wires and use it for the operation of various devices. The current strength together with the cell voltage determines the power of the electricity produced by the photocell.
Solar panel ©depositphotos.com
Silicon semiconductors
Consider the process of releasing electrons using the example of silicon. A silicon atom has 14 electrons in three shells. The first two shells are completely filled with two and eight electrons, respectively. The third shell is half empty, with only 4 electrons.
Because of this, silicon has a crystalline shape; trying to fill the voids in the third shell, silicon atoms try to “share” electrons with neighbors. However, a crystal of silicon in its pure form is a poor conductor, since almost all of its electrons sit firmly in a crystal lattice.
Therefore, solar panels do not use pure silicon, but crystals with small impurities, that is, atoms of other substances are introduced into silicon. There is only one atom per million silicon atoms, such as a phosphorus atom.
Phosphorus has five electrons in its outer shell. Four of them form crystalline bonds with nearby silicon atoms, but the fifth electron is actually left hanging in space, without any bonds with neighboring atoms.
When sunlight hits silicon, its electrons receive additional energy, which is enough to tear them away from the corresponding atoms. As a result, “holes” remain in their place. The freed electrons wander through the crystal lattice as carriers of electric current. When they meet another hole, they fill it.
However, in pure silicon, such free electrons are too few due to the strong bonds of atoms in the crystal lattice. Quite another thing - silicon with an admixture of phosphorus. The release of unbound electrons in phosphorus atoms requires much less energy.
Most of these electrons become free carriers that can be efficiently directed and used to generate electricity. The process of adding impurities to improve the chemical and physical properties of a substance is called doping.
Silicon doped with phosphorus atoms becomes an n-type electronic semiconductor (from the word "negative", due to the negative charge of electrons).
Silicon is also doped with boron, which has only three electrons in its outer shell. The result is a p-type semiconductor (from “positive”), in which free positively charged “holes” arise.
Solar battery device
So what happens when you connect an n-type semiconductor to a p-type semiconductor? In the first of them, many free electrons were formed, and in the second, many holes. Electrons tend to fill the holes as quickly as possible, but if that happens, both semiconductors will become electrically neutral.
Instead, when free electrons enter a p-type semiconductor, the region at the junction of both substances is charged, forming a barrier that is not so easy to cross. At the p-n boundary of the transition there is an electric field.
The energy of each photon of sunlight is usually enough to release one electron, and therefore to form one extra hole. If this happens near the p-n junction, the electric field sends the free electron to the n-side and the hole to the p-side.
Thus, the equilibrium is disturbed even more, and if an external electric field is applied to the system, free electrons will flow to the p-side to fill the holes, creating an electric current.
Unfortunately, silicon reflects light quite well, which means that a significant part of the photons disappear in vain. To reduce losses, solar cells are coated with anti-reflective coating. Finally, to protect the solar battery from rain and wind, it is also customary to cover it with glass.

The world's largest solar-powered ship PlanetSolar ©PlanetSolar/Philip Plisson
The efficiency of modern solar cells is not too high. Most of them efficiently process 12 to 18 percent of the sunlight they receive. The best samples crossed the 40% efficiency barrier.
Source: naked-science.ru/article/nakedscience/how-solar-cells-work