1017
Amazing chemical reactions
Surely there will be among the readers of this post are people who doted in Chemistry during his school years. Indeed: blow bulb dissolving desks and painted themselves from head to toe was incredibly fun. I therefore propose to look at the most fascinating chemical reactions in a unique collection gifok. Read on!

Chemical reactions
1. "Pharaoh snake" - the collapse of the mercury thiocyanate

Burning mercury thiocyanate leads to its decomposition into three other chemical substances. These three chemicals in turn decomposed into three substances, which leads to an enormous deployment \ "snake \».
2. Burning match
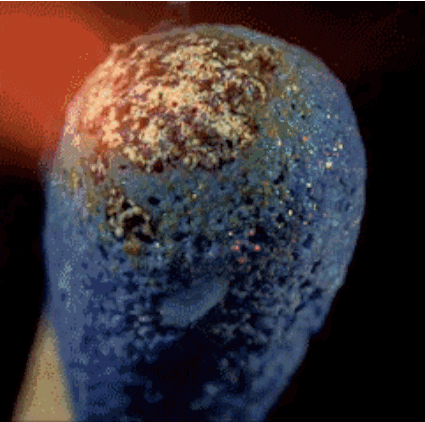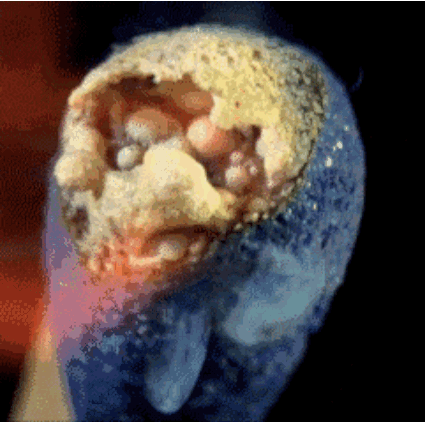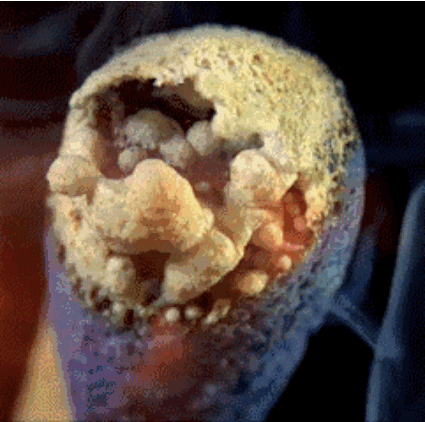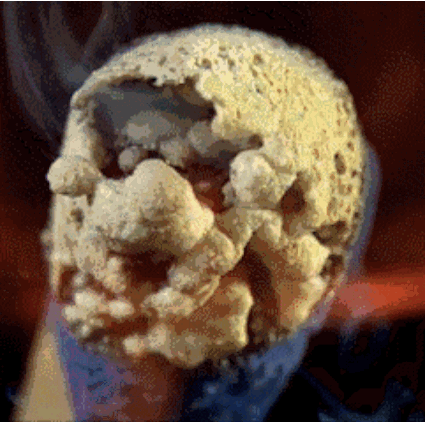
Match head contains red phosphorus, sulfur and potassium chlorate salt. Heat generated by the phosphorus salt and potassium chlorate decompose during releases oxygen. Oxygen in combination with sulfur produces short flames, which we use to light, such as a candle.
3. Fire + hydrogen

Hydrogen gas is lighter than air and can ignite a flame or spark, leading to a dramatic explosion. That is why now more commonly used helium, rather than hydrogen filling balloons.
4. Mercury + aluminum

Mercury penetrates the protective layer of oxide (rust) of aluminum, causing it to corrode much faster.
[color = # 3333FF] Examples of chemical reactions [/ color]
5. Snake venom + blood

One drop of viper venom that gets into a petri dish with blood, causes her to curl into a thick clump solid. This is what happens in our bodies when we are bitten by a poisonous snake.
6. Iron + copper sulfate solution
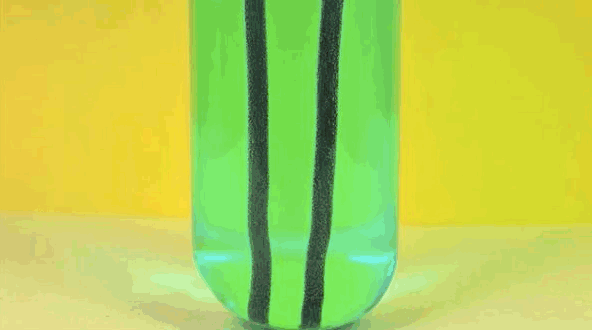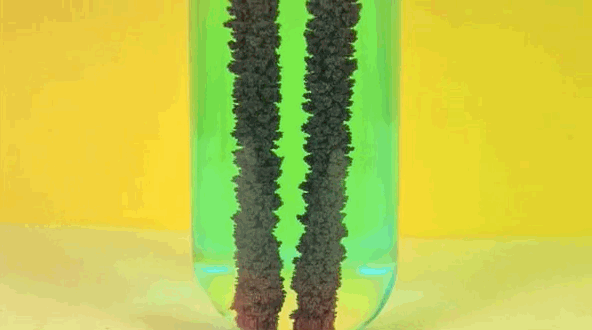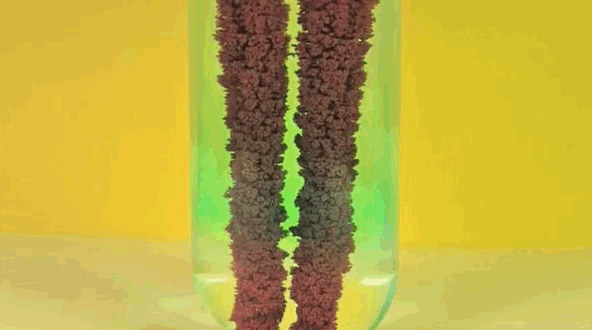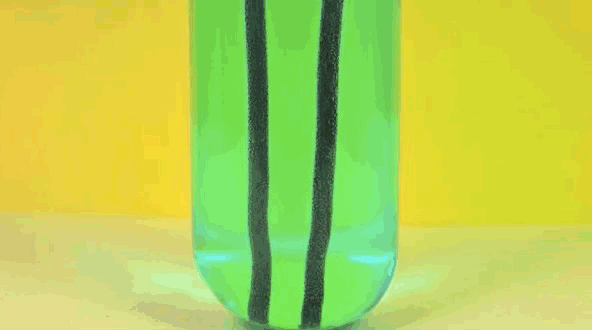
Iron replaces copper in solution, making bluestone in vitriol. Pure copper is going on iron.
7. Ignition gas bottle

8. chlorine tablets + rubbing alcohol in a closed bottle

The reaction leads to an increase in pressure and rupture the container ends.
9. Polymerization of p-nitroaniline

On SFII to half teaspoon of p-nitroaniline or 4-nitroaniline are added several drops of concentrated sulfuric acid.
10. Blood hydrogen peroxide

The enzyme in the blood, called Catalase converts hydrogen peroxide into water and oxygen gas, oxygen bubbles create foam.
Chemical experiments
11. Gallium in hot water

Gallium, which is mainly used in electronics, has a melting point of 29, 4 degrees Celsius, and therefore will melt in their hands.
12. The slow transition beta-tin alpha modification
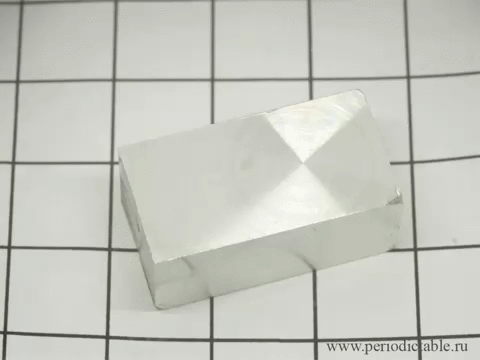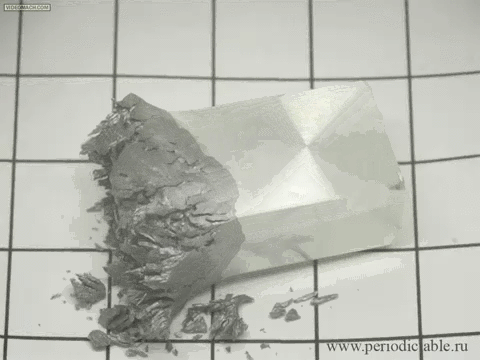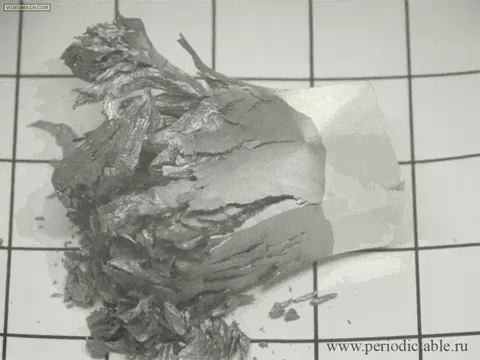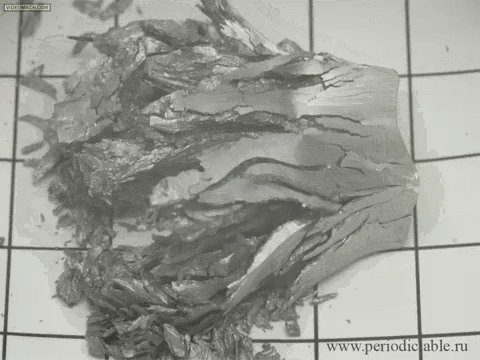
In cold temperatures beta allotrope of tin (silver, metallic) spontaneously goes into alpha allotrope (gray powder).
13. Sodium polyacrylate + water

Sodium polyacrylate - the same material that is used in baby diapers, acts like a sponge, soaking up moisture. When mixed with water, the compound is converted into a solid gel while the water is no longer liquid and can not be poured.
14. 220 radon gas is injected into the misty chamber

Traces of the V-shaped occur because of two alpha particles (helium nuclei-4), which are released when radon decays into polonium and then lead.
Household chemical experiments
15. The hydrogel beads and multi-colored water

In this case, the diffusion effect. A hydrogel is a polymer beads that absorb water very well.
16. Acetone + foam

Foam consists of polystyrene, which when dissolved in acetone, releases air into the foam, which creates a look as if you dissolve a large amount of material in a small amount of liquid.
17. Dry ice + dishwashing detergent

Dry ice is placed in the water creates a cloud, and dishwashing detergent in water keeps the carbon dioxide and water vapor in the form of a bubble.
18. A drop of detergent added to the milk with food coloring
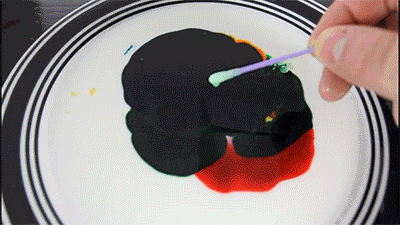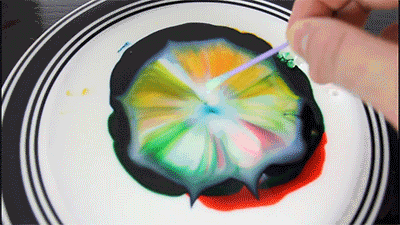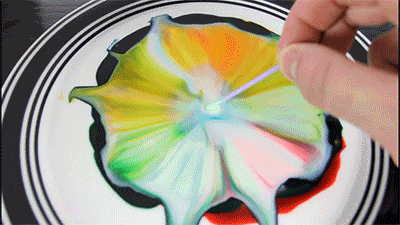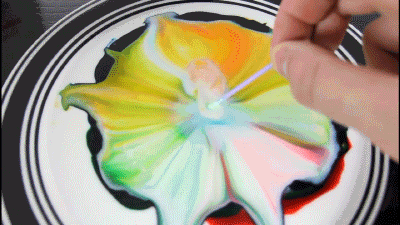
Milk - is mainly water, but it also contains vitamins, minerals, proteins, and tiny drops of fat that are suspended in the solution.
Dishwashing detergent weaken the chemical bonds that hold the proteins and fats in the solution. The molecules of fat confusing for as soap molecules begin to rush to connect with molecules of fat, while the solution will mix evenly.
19. \ "Elephant Toothpaste \»

Yeast and warm water is poured into the container with detergent, hydrogen peroxide and food coloring. Yeast are a catalyst for the release of oxygen from hydrogen peroxide, creating a lot of bubbles. The result is an exothermic reaction, with the formation of foam and heat release.
20. A blown bulb

The tungsten filament is broken, causing a short circuit electrical circuit that causes the filament to glow.
21. ferrofluid in a glass jar

Ferromagnetic fluid - is a liquid which is strongly magnetized in a magnetic field. It is used in hard disk drives, and in engineering.
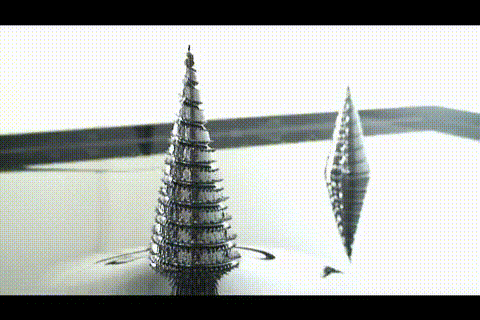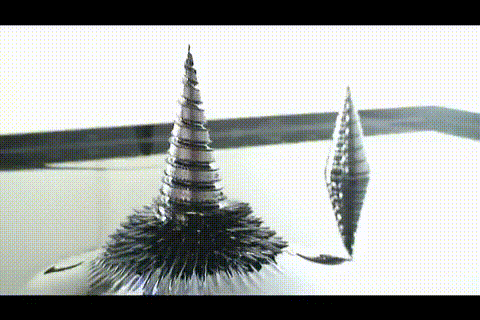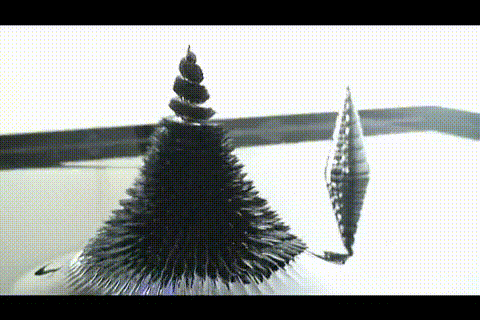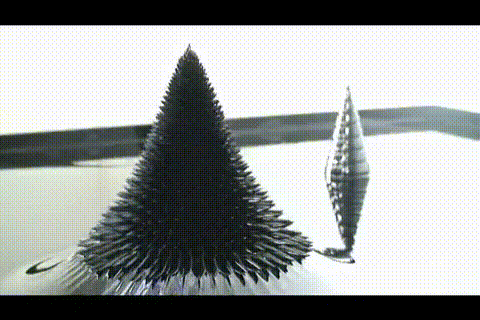
More ferrofluid.
22. Iodine + aluminum
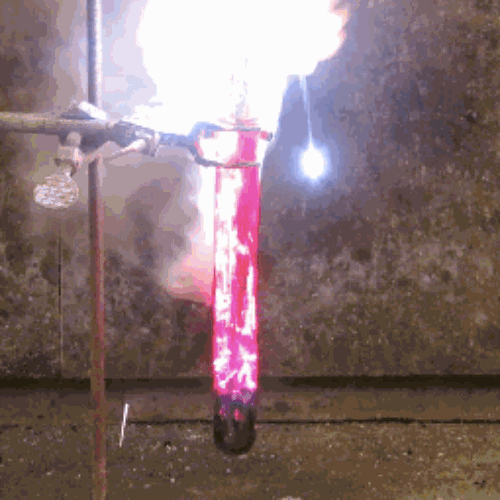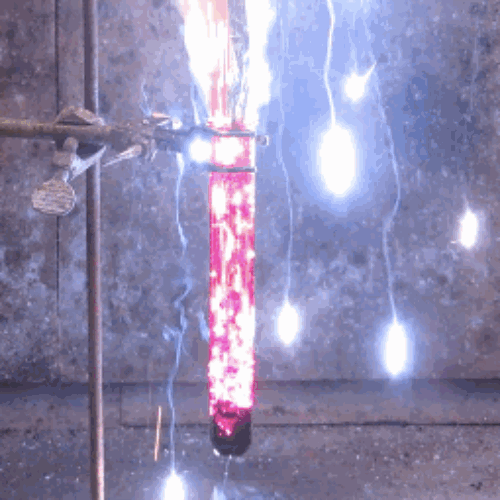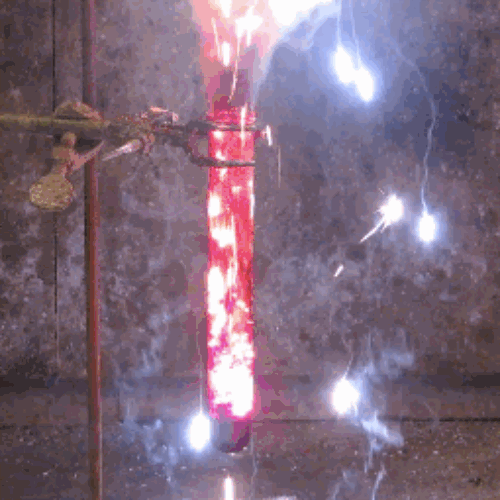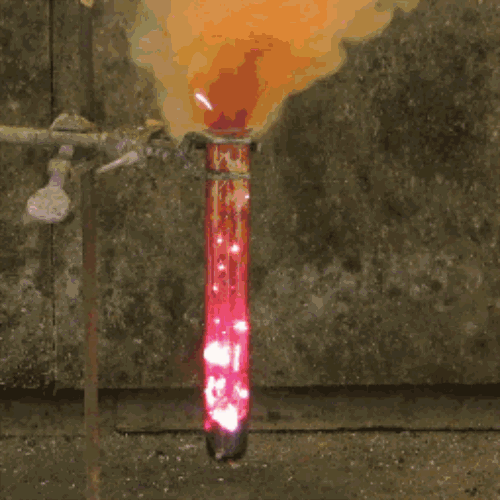
The oxidation of finely divided aluminum occurs in water to form a dark purple pair.
23. Rubidium + water

Rubidium rapidly reacts with water, forming a rubidium hydroxide and hydrogen gas. The reaction is so fast that if it were carried out in a glass vessel, it may break.

Chemical reactions
1. "Pharaoh snake" - the collapse of the mercury thiocyanate

Burning mercury thiocyanate leads to its decomposition into three other chemical substances. These three chemicals in turn decomposed into three substances, which leads to an enormous deployment \ "snake \».
2. Burning match

Match head contains red phosphorus, sulfur and potassium chlorate salt. Heat generated by the phosphorus salt and potassium chlorate decompose during releases oxygen. Oxygen in combination with sulfur produces short flames, which we use to light, such as a candle.
3. Fire + hydrogen

Hydrogen gas is lighter than air and can ignite a flame or spark, leading to a dramatic explosion. That is why now more commonly used helium, rather than hydrogen filling balloons.
4. Mercury + aluminum

Mercury penetrates the protective layer of oxide (rust) of aluminum, causing it to corrode much faster.
[color = # 3333FF] Examples of chemical reactions [/ color]
5. Snake venom + blood

One drop of viper venom that gets into a petri dish with blood, causes her to curl into a thick clump solid. This is what happens in our bodies when we are bitten by a poisonous snake.
6. Iron + copper sulfate solution
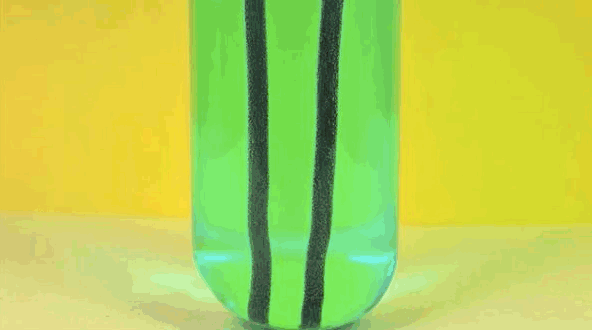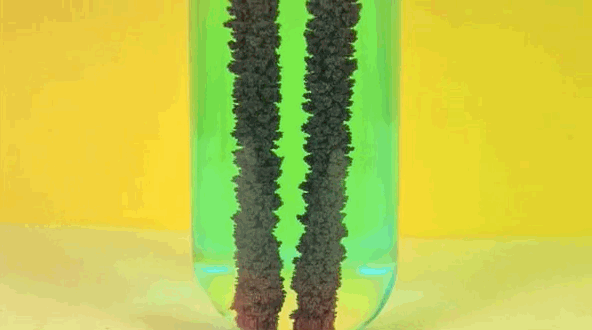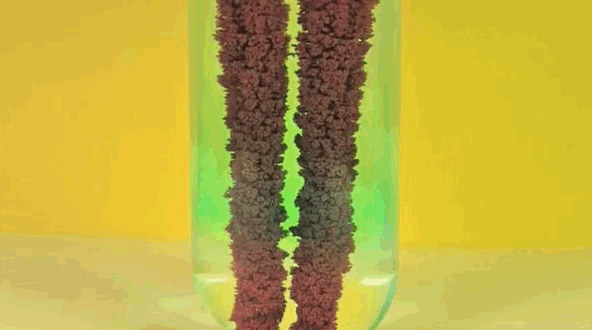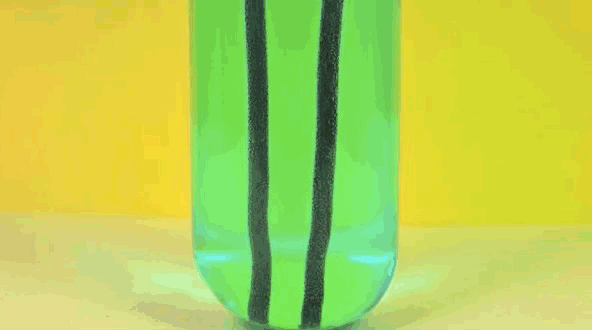
Iron replaces copper in solution, making bluestone in vitriol. Pure copper is going on iron.
7. Ignition gas bottle

8. chlorine tablets + rubbing alcohol in a closed bottle

The reaction leads to an increase in pressure and rupture the container ends.
9. Polymerization of p-nitroaniline

On SFII to half teaspoon of p-nitroaniline or 4-nitroaniline are added several drops of concentrated sulfuric acid.
10. Blood hydrogen peroxide

The enzyme in the blood, called Catalase converts hydrogen peroxide into water and oxygen gas, oxygen bubbles create foam.
Chemical experiments
11. Gallium in hot water

Gallium, which is mainly used in electronics, has a melting point of 29, 4 degrees Celsius, and therefore will melt in their hands.
12. The slow transition beta-tin alpha modification

In cold temperatures beta allotrope of tin (silver, metallic) spontaneously goes into alpha allotrope (gray powder).
13. Sodium polyacrylate + water

Sodium polyacrylate - the same material that is used in baby diapers, acts like a sponge, soaking up moisture. When mixed with water, the compound is converted into a solid gel while the water is no longer liquid and can not be poured.
14. 220 radon gas is injected into the misty chamber

Traces of the V-shaped occur because of two alpha particles (helium nuclei-4), which are released when radon decays into polonium and then lead.
Household chemical experiments
15. The hydrogel beads and multi-colored water

In this case, the diffusion effect. A hydrogel is a polymer beads that absorb water very well.
16. Acetone + foam

Foam consists of polystyrene, which when dissolved in acetone, releases air into the foam, which creates a look as if you dissolve a large amount of material in a small amount of liquid.
17. Dry ice + dishwashing detergent

Dry ice is placed in the water creates a cloud, and dishwashing detergent in water keeps the carbon dioxide and water vapor in the form of a bubble.
18. A drop of detergent added to the milk with food coloring

Milk - is mainly water, but it also contains vitamins, minerals, proteins, and tiny drops of fat that are suspended in the solution.
Dishwashing detergent weaken the chemical bonds that hold the proteins and fats in the solution. The molecules of fat confusing for as soap molecules begin to rush to connect with molecules of fat, while the solution will mix evenly.
19. \ "Elephant Toothpaste \»

Yeast and warm water is poured into the container with detergent, hydrogen peroxide and food coloring. Yeast are a catalyst for the release of oxygen from hydrogen peroxide, creating a lot of bubbles. The result is an exothermic reaction, with the formation of foam and heat release.
20. A blown bulb

The tungsten filament is broken, causing a short circuit electrical circuit that causes the filament to glow.
21. ferrofluid in a glass jar

Ferromagnetic fluid - is a liquid which is strongly magnetized in a magnetic field. It is used in hard disk drives, and in engineering.

More ferrofluid.
22. Iodine + aluminum

The oxidation of finely divided aluminum occurs in water to form a dark purple pair.
23. Rubidium + water

Rubidium rapidly reacts with water, forming a rubidium hydroxide and hydrogen gas. The reaction is so fast that if it were carried out in a glass vessel, it may break.