402
Engineers accidentally increased the battery life a few hundred times
Engineers from the University of California, Irvine, trying to create a solid-state battery, suddenly received the cathodes to withstand a couple orders of magnitude more recharge cycles than normal. The cathodes consisted of gold nanowires covered with gel.
The cathodes obtained by scientists stood 200000 recharge cycles without significant corrosion. Loss of capacity compared to the first cycles was not more than 5%. Conventional lithium-ion batteries can withstand several thousand cycles. Scientists do not yet know how their design can withstand such loads: they were just trying to create a battery, where liquid electrolyte is used gel.

Since traditional batteries are sensitive to temperature and can explode, engineers are looking for a replacement. But while much progress has not been achieved – liquid has high conductivity and allows the partial charge and discharge.
The cathode consists of a gold nanowire covered with oxide of manganese and protected by a layer of gel that is used as the electrolyte. The gel protects the wire from corrosion. Battery capacity is proportional to the length of the wire. This is not a unique design, with the exception of the gel, which gives it unusual properties.
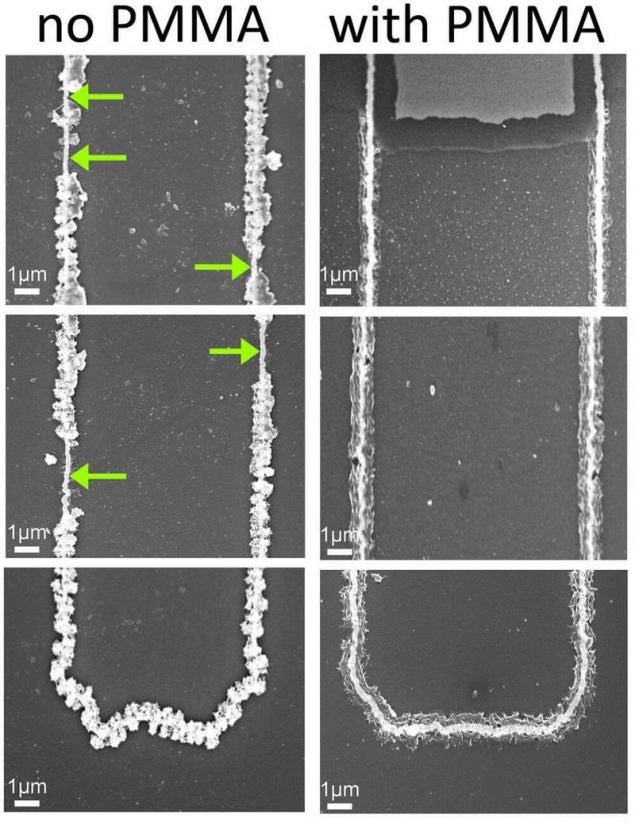
"We started to test the recharge cycles, and at some point he found that the battery is not going to die - says the opening of the Reginald Penner [Penner Reginald], lead author of the work. Gel not just does not allow the wire to fall apart – it looks like it attaches to the oxide of the metal additional softness and protects it from damages. It increases the strength of the metal oxide".
While scientists have not created a full battery, and tested only the material for the anode. In addition, the imminent industrial application of the technology separates the cost of production of microscopic amounts of gold used to create nanowires, it is still expensive construction.
Penner believes that there may be suitable other metal, e.g., Nickel. While scientists are planning to create a comprehensive battery to assess the performance of their new designs in conditions close to real. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/274807/
The cathodes obtained by scientists stood 200000 recharge cycles without significant corrosion. Loss of capacity compared to the first cycles was not more than 5%. Conventional lithium-ion batteries can withstand several thousand cycles. Scientists do not yet know how their design can withstand such loads: they were just trying to create a battery, where liquid electrolyte is used gel.

Since traditional batteries are sensitive to temperature and can explode, engineers are looking for a replacement. But while much progress has not been achieved – liquid has high conductivity and allows the partial charge and discharge.
The cathode consists of a gold nanowire covered with oxide of manganese and protected by a layer of gel that is used as the electrolyte. The gel protects the wire from corrosion. Battery capacity is proportional to the length of the wire. This is not a unique design, with the exception of the gel, which gives it unusual properties.

"We started to test the recharge cycles, and at some point he found that the battery is not going to die - says the opening of the Reginald Penner [Penner Reginald], lead author of the work. Gel not just does not allow the wire to fall apart – it looks like it attaches to the oxide of the metal additional softness and protects it from damages. It increases the strength of the metal oxide".
While scientists have not created a full battery, and tested only the material for the anode. In addition, the imminent industrial application of the technology separates the cost of production of microscopic amounts of gold used to create nanowires, it is still expensive construction.
Penner believes that there may be suitable other metal, e.g., Nickel. While scientists are planning to create a comprehensive battery to assess the performance of their new designs in conditions close to real. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Join us in Facebook , Vkontakte, Odnoklassniki
Source: geektimes.ru/post/274807/
Russian police in the near future will be able to remotely disable the engine of any car
As for the eye to determine the batch sizes of products