440
Negligence has enabled researchers to increase the service life of the batteries 4 times
Leading Chinese scientists of the Tsinghua University and the U.S. Massachusetts Institute of technology led a long and painstaking work on extending the service life of lithium-ion batteries. However, the desired result, the researchers brought a negligence. The party of anodes by accident remained under chemical treatment for a few hours longer than necessary. As a result, their strength has increased four times...

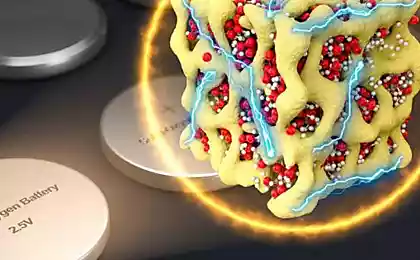
The degradation of the anode material is the main reason for the reduction in battery life. Several hundred cycles of charge and discharge significantly affect durability of the battery. For anodes of lithium-ion battery cells, the researchers decided to use aluminum instead of graphite, although it expands and contracts stronger. Because of this, the destruction of the material occurs faster. The researchers decided to stop the formation of the oxide coating on aluminum nanoparticles by soaking the materials in sulphuric acid and oxide of titanium sulfate. It replaces the aluminium oxide more conductive titanium oxide.
One of the scientists accidentally left to soak the anodes in the chemical solution for several hours longer than necessary. As a result, the party was spoiled. Fortunately, the researchers did not think to throw it away, and decided to check. It turned out that the new anodes have a shell of solid nanoparticles that enclose the aluminum material. It expands and contracts without damaging the external coating. This design is quadrupling the number of cycles of charge-discharge batteries. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: vk.com/wiki_inventions

The degradation of the anode material is the main reason for the reduction in battery life. Several hundred cycles of charge and discharge significantly affect durability of the battery. For anodes of lithium-ion battery cells, the researchers decided to use aluminum instead of graphite, although it expands and contracts stronger. Because of this, the destruction of the material occurs faster. The researchers decided to stop the formation of the oxide coating on aluminum nanoparticles by soaking the materials in sulphuric acid and oxide of titanium sulfate. It replaces the aluminium oxide more conductive titanium oxide.
One of the scientists accidentally left to soak the anodes in the chemical solution for several hours longer than necessary. As a result, the party was spoiled. Fortunately, the researchers did not think to throw it away, and decided to check. It turned out that the new anodes have a shell of solid nanoparticles that enclose the aluminum material. It expands and contracts without damaging the external coating. This design is quadrupling the number of cycles of charge-discharge batteries. published
P. S. And remember, only by changing their consumption — together we change the world! ©
Source: vk.com/wiki_inventions