Electric field to generate environmentally friendly electricity
 Bashny.Net
Bashny.Net
Characteristics of compounds produced by burning fuel can be the key to cleaner and more efficient power plants.
Modeling of the combustion of fossil fuels by KAUST researchers helped to characterize some of the components methane and to lay the foundations for the production of clean energy.

In the production of energy, nedorogaya fossil fuels , such as natural gas leads to emissions of polluting substances into the atmosphere. This is such substances as soot, carbon monoxide and nitrogen oxides, all of them are harmful for our health and the environment.
The application of an external electric field to control the combustion process, as is known, reduces the formation of these pollutants, but the mechanism of this is not fully understood. It made Amir Farouk and his colleagues from research center for clean combustion KAUST to develop a catalogue of compounds formed during the combustion of methane, the main component of natural gas, which can lead to the development of more efficient gas turbines and more environmentally friendly power plants.
"Control of charged particles creates a more compact, more enriched air-fuel mixture, combustion occurs at lower temperatures, providing a more complete and efficient combustion and reduce the formation of pollutants," explains Farooq.
Positively charged particles, called cations, are formed when the electrons leave their atoms in the combustion process. They are less common, often value many orders of magnitude different from the neutral molecules, which makes the observation of the formation and povedenie of these ions is a complicated technical task.
To overcome these difficulties, researchers have used the McKenna burner to obtain a stable, low pressure methane-oxygen-argon flame. They used a specially designed molecular beam spectrometer to measure the ions formed in the flame, which allowed to distinguish them from the more visible neutral (uncharged) compounds.
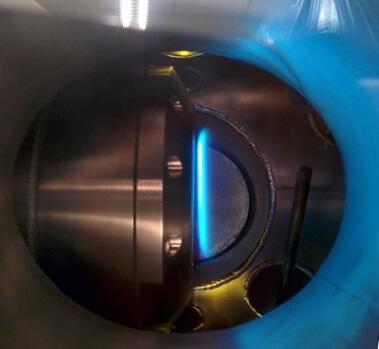
"We are able to measure a wide range of ions, enabling us to understand how they are formed and decay that lead to the formation of other ions," explains Farooq.
The presence of hydronium indicates the acidity of the flames, but in more acidic the flame, there are favorable conditions for the formation of soot particles; therefore, the measurement of its relative concentration is crucial to reduce the level of contamination with soot.
"Our work is the first attempt to determine the cations generated in the combustion of methane at low pressure, and represents an important step towards the use of external electric fields to generate cleaner electricity," says Farooq. published
Source: ecotechnology
Modeling of the combustion of fossil fuels by KAUST researchers helped to characterize some of the components methane and to lay the foundations for the production of clean energy.

In the production of energy, nedorogaya fossil fuels , such as natural gas leads to emissions of polluting substances into the atmosphere. This is such substances as soot, carbon monoxide and nitrogen oxides, all of them are harmful for our health and the environment.
The application of an external electric field to control the combustion process, as is known, reduces the formation of these pollutants, but the mechanism of this is not fully understood. It made Amir Farouk and his colleagues from research center for clean combustion KAUST to develop a catalogue of compounds formed during the combustion of methane, the main component of natural gas, which can lead to the development of more efficient gas turbines and more environmentally friendly power plants.
"Control of charged particles creates a more compact, more enriched air-fuel mixture, combustion occurs at lower temperatures, providing a more complete and efficient combustion and reduce the formation of pollutants," explains Farooq.
Positively charged particles, called cations, are formed when the electrons leave their atoms in the combustion process. They are less common, often value many orders of magnitude different from the neutral molecules, which makes the observation of the formation and povedenie of these ions is a complicated technical task.
To overcome these difficulties, researchers have used the McKenna burner to obtain a stable, low pressure methane-oxygen-argon flame. They used a specially designed molecular beam spectrometer to measure the ions formed in the flame, which allowed to distinguish them from the more visible neutral (uncharged) compounds.

"We are able to measure a wide range of ions, enabling us to understand how they are formed and decay that lead to the formation of other ions," explains Farooq.
The presence of hydronium indicates the acidity of the flames, but in more acidic the flame, there are favorable conditions for the formation of soot particles; therefore, the measurement of its relative concentration is crucial to reduce the level of contamination with soot.
"Our work is the first attempt to determine the cations generated in the combustion of methane at low pressure, and represents an important step towards the use of external electric fields to generate cleaner electricity," says Farooq. published
Source: ecotechnology
Tags
See also
Hyundai can make a "smart" hours on the Android Wear
Graphene coating to improve the condensation of steam will help increase the efficiency of power plants
The ultra-fast heating up to the temperature of the Sun
Building a house of expanded-clay concrete blocks
The most unexpected ways to use colas
Other ways to use colas
Warm floor for a country house
5 "green" technologies, because of which we do not understand why petrol is still popular
A masterpiece of industrial architecture (43 pics + text)
Renewable sources is not enough. Clean Coal - Energy of the near future

















