Solar panels for the production of hydrogen
 Bashny.Net
Bashny.Net
One of the potential clean energy of the future is economical, efficient and relatively simple way to create copious amounts of hydrogen, which is then used in fuel cells and hydrogen vehicles.
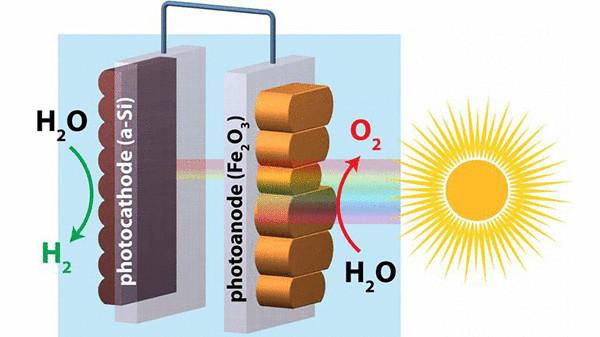
This process often occurs with electricity, splitting water molecules into hydrogen and oxygen — but the ideal solution would be to extract hydrogen from water with electricity generated directly from sunlight without the addition of any external energy source.
Hematite is a mineral form of iron used in conjunction with silicon promised some progress in this area, but research has shown low conversion efficiency. Now scientists have found a way to make significant improvements using two of the most common elements on Earth, promising efficient production of hydrogen.
Hematite has the potential for use in low-power photoelectrochemical splitting of water (where the input light energy and chemical energy output) with the release of hydrogen due to its low activation voltage less than 0.3 volts when exposed to sunlight. Unfortunately this voltage is too low to initiate the splitting of water, therefore, for improved electrical current flow to the hematite surface was applied a few improvements.
In this regard, researchers from Boston College, the University of California at Berkeley, and Chinese University of Science and Technology suddenly found the technology "re-building" of hematite, so the material is a smooth surface along with a higher energy output. In fact, this new version has doubled the electrical power, and approached a step closer to practical applications: large-scale hydrogen production.
"Just smoothing out the surface characteristics of hematite, it can be upgraded to allow communication with the silicon, which is derived from sand, to achieve full splitting water to produce hydrogen," said Professor of chemistry from Boston College, Danwei Wang (Dunwei Wang). "This method of splitting water is completely self-contained, does not require expensive or scarce resources".
Working on a previous project, which aims to maximize the efficiency in terms of voltage photoelectrochemical activation when using smooth surfaces, the team re-evaluated the structure of the surface of hematite using a synchrotron particle accelerator at the National laboratory Lawrence Berkeley.
Focusing on the processing and surface defects of hematite, they decided to check whether this will lead to improvement. The researchers used a vacuum the imposition of a layer of hematite on a substrate of borosilicate glass, and created a photoanode. Then they dried the device for producing thin films of iron oxide across the surface.
Subsequent tests of this new amalgam led to an immediate improvement in the voltage activation and a significant increase of the photovoltage from 0.24 to 0.80 volts. Although this new process of collecting hydrogen has an efficiency of only 0.91%, this is the first time a combination of hematite and amorphous silicon have shown any significant efficiency at all.
As a result, this study showed progress in the direction of the possibility of photoelectrochemical energy harvesting, which is completely self-sufficient, uses available materials, and easy to manufacture.
"This gives hope for new effective and low-cost solar fuel production using readily available natural resources," said Wang. "The use of this technology will contribute to a sustainable future energy supply from renewable energy sources". .published
P. S. And remember, just changing your mind - together we change the world! © econe
Source: www.ekopower.ru/?p=3601

This process often occurs with electricity, splitting water molecules into hydrogen and oxygen — but the ideal solution would be to extract hydrogen from water with electricity generated directly from sunlight without the addition of any external energy source.
Hematite is a mineral form of iron used in conjunction with silicon promised some progress in this area, but research has shown low conversion efficiency. Now scientists have found a way to make significant improvements using two of the most common elements on Earth, promising efficient production of hydrogen.
Hematite has the potential for use in low-power photoelectrochemical splitting of water (where the input light energy and chemical energy output) with the release of hydrogen due to its low activation voltage less than 0.3 volts when exposed to sunlight. Unfortunately this voltage is too low to initiate the splitting of water, therefore, for improved electrical current flow to the hematite surface was applied a few improvements.
In this regard, researchers from Boston College, the University of California at Berkeley, and Chinese University of Science and Technology suddenly found the technology "re-building" of hematite, so the material is a smooth surface along with a higher energy output. In fact, this new version has doubled the electrical power, and approached a step closer to practical applications: large-scale hydrogen production.
"Just smoothing out the surface characteristics of hematite, it can be upgraded to allow communication with the silicon, which is derived from sand, to achieve full splitting water to produce hydrogen," said Professor of chemistry from Boston College, Danwei Wang (Dunwei Wang). "This method of splitting water is completely self-contained, does not require expensive or scarce resources".
Working on a previous project, which aims to maximize the efficiency in terms of voltage photoelectrochemical activation when using smooth surfaces, the team re-evaluated the structure of the surface of hematite using a synchrotron particle accelerator at the National laboratory Lawrence Berkeley.
Focusing on the processing and surface defects of hematite, they decided to check whether this will lead to improvement. The researchers used a vacuum the imposition of a layer of hematite on a substrate of borosilicate glass, and created a photoanode. Then they dried the device for producing thin films of iron oxide across the surface.
Subsequent tests of this new amalgam led to an immediate improvement in the voltage activation and a significant increase of the photovoltage from 0.24 to 0.80 volts. Although this new process of collecting hydrogen has an efficiency of only 0.91%, this is the first time a combination of hematite and amorphous silicon have shown any significant efficiency at all.
As a result, this study showed progress in the direction of the possibility of photoelectrochemical energy harvesting, which is completely self-sufficient, uses available materials, and easy to manufacture.
"This gives hope for new effective and low-cost solar fuel production using readily available natural resources," said Wang. "The use of this technology will contribute to a sustainable future energy supply from renewable energy sources". .published
P. S. And remember, just changing your mind - together we change the world! © econe
Source: www.ekopower.ru/?p=3601
Tags
See also
Developed solar cells that are twice as efficient as conventional
Solar battery shrimp
Transparent solar panels for tablets
Sunny forest for electromobile
Scientists have turned Windows into solar panels
Tesla and Panasonic will work together to produce solar panels
Tesla has turned a tropical island into a solar cell
The list of the most efficient solar panels
Comparative test of solar panels: flexible vs rigid

















