The half-century mystery intermediate carbon structure solved: this M-carbon
 Bashny.Net
Bashny.Net
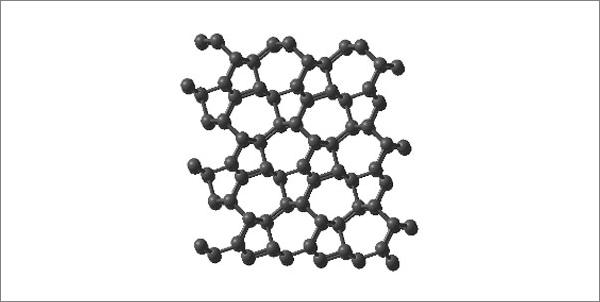
Physics from Yale University (USA) for the first time confirmed the theoretically predicted structure carbon cold pressure (carbon modifications comparable in hardness to diamond), performed experiments on compression of graphite at high pressure and room temperature.
The researchers believe that their achievement will in future receive the superhard material, which is able to withstand a lot of pressure and can serve as an alternative used today in electronics and industrial diamond materials. Report on the work published in the open web journal Scientific Reports.
Under normal conditions, pure carbon is in the form of various modifications that have completely different physical properties, which are determined by their structure. For example, graphite - soft, conductive substance, and a diamond - insulator and one of the hardest materials known to man. And somewhere in the middle between these modifications is widespread natural theoretically predicted in 2006 the structure of the so-called M-carbon, the existence of which could be confirmed by Yale scientists.
M-carbon was obtained from an ordinary graphite at room temperature under a pressure of 200 000 atmospheres. By the way, there is a theoretical "intermediate" diamond structure was proposed several alternative structures, one of which is M-carbon. His existence and that was confirmed in practice, and all the rest you can now recognize erroneous.
In fairness, we recall that the first changes that occur with graphite at high pressure and room temperature, were seen 50 years ago, but only now crystal structure was fully confirmed experimentally using X-ray diffraction, Raman spectroscopy, and various optical methods. In addition to the unique mechanical properties of M-carbon, it was found that the transformation of graphite in the M-carbon occurs extremely slowly, requiring a very long time to achieve the desired balance, which appears to be an important reason, is not allowed to get this phase of half a century ago. < br />
Scientists say that they obtained an intermediate structure is characterized by a much lower symmetry than diamond, but it is very, very hard. Studies have shown that M-carbon - extremely incompressible substance that can scratch diamond.
Tags
See also
Stanley Milgram: what is the measure of "obedience" inherent in man
7 reasons to do fitness
Look for THEIR
How to make a greenhouse according to the method of Mittleider
The theories of the universe
6 of the most incredible and amusing theories about the structure of the universe
Six theories about the structure of our world, that can be true
What is behind these letters?
13 unstudied phenomena

















